- Submissions

Full Text
Archaeology & Anthropology:Open Access
Evaluation of Marble Structural Damage due to Rain Anions
Margarita Teutli León1*, Andres Sánchez Hernandez2 and César Márquez Beltrán3
1Facultad de Ingeniería, Mexico
2Facultad de Arquitectura, Mexico
3Facultad de Ciencias Químicas, Mexico
*Corresponding author: Margarita Teutli León, Facultad de Ingeniería, Benemérita Universidad Autónoma de Puebla (BUAP), Mexico
Submission: March 13, 2020Published: June 03, 2020

ISSN: 2577-1949 Volume3 Issue5
Abstract
In this paper it is reported an experimental approach for assessing marble response to the activity of rain anions. For this study it was considered to extract a nucleus from a marble stone, divided into slabs which were placed into 0.1M solutions of HCl, CaCO3, H2SO4 and distilled water as a blank. Weathering evolution was followed throughout register of: slab mass weight, solution pH and electrical conductivity, plus some images were taken from the top of each slab with an inverse stage microscopy.
Results have provided evidence that the most aggressive anion was chloride since it produces the biggest weight loss, and although sulfate favors weight gain it produces marble degradation by forming gypsum and fracturing the structure. Also it became evident that pH solution trend is close to neutrality for HCl, CaCO3 and H2O, but H2SO4 goes to acidic values, also electrical conductivity have an inverse correlation in the case of sulfate.
Keywords: Marble; Weathering; Chloride; Sulfate; Carbonate
Introduction
Evaluation of marble structural damage due to rain anions
Stone material exhibits different degree of sensibility to weather factors like thermal variations, solar radiation, water cycle processes, wind erosion, solar radiation; although all mentioned factors do no act by itself. In the case of carbonate stones like marble or sandy stone these become structurally destroyed by interaction with water in its different forms and process, for instance: vapor condensation, freezing, thawing, and salt crystallization/ dissolution within pores.
Marble and limestone are common stones used in historic structures; both suffer deterioration from physical chemical and biological causes. Damage become evident in different ways like surface deposits, discoloration, pitting, accelerated weathering, but before suggesting a treatment it is important to determine what kind of microorganism is present (bacteria, fungi, algae, actynomicetes) as well as the minimal inhibitory concentration (MIC) of antimicrobial agents, Abdelhafez et al. [1] reported that 100ppm of sodium azide works fine on most of the isolated microorganisms.
Stone pieces immersed in water suffer deterioration mainly from biological colonization Cámara et al. [2], performed a study on Carrara ( fine grained calcite) and Macael (medium to coarse calcite grains) marbles exposed to seawater their experiments provided an estimation of surface biocover. Damage characterization was done using polarized light microscopy (PLM), X-ray powder diffraction (XRD), X-ray fluorescence (XRF) and optical surface roughness (OSR). Authors conclude that the biological colonization was more related to the underwater conditions than to the intrinsic properties of the marble studied.
Solar weathering of marble has been studied by Eppes & Griffing [3], authors used as reference a natural landscape at San Bernardino Mountains in California, authors found that marble corestone formation and granular disintegration is mainly occurring in the subaerial rock surface due to cohesion loss due to thermos-mechanical processes taking place at the grain boundaries and rates of erosion due to physical weathering exceeds those due to chemical dissolution alone, because there is minimal evidence of secondary calcite dissolution/precipitation at grain boundaries.
It has been reported [4] that marble exposed to the environment exhibit deformation or fractures, these damages are closely related to dominant weather conditions like temperature and humidity. At long term the alternating stresses conditions (hot/cold, wet/dry, frozen/thawing) are driving forces in developing stone damage. For rocks exposed to the environment, their structural damage and degradation have been reported in the literature, these reports point out that the deterioration process takes place in 3 phases:
Phase I: Variation in temperature and stress produce damage due to decohesion of micro crystals, these alterations produces a change in pore structure allowing for a slow water penetration into submicropores (diameter<4nm). Induction of structural damage.
Phase II: Temperature changes produce a slow expansion of grain borders, so far the porosity increases, making more fragile the rock.
The occurrence of these two phases are responsible for the basic damage in marble, variations in intensity can take place due to position and orientation.
Phase III: This phase depends on local temperature and humidity; inside the pores, water absorption produces structural damage, which at long time becomes evident as marble destruction.
Although structural destruction of marble is important, few studies have contributed to identify the required time for each phase to take place.
Some of the most recent methods to examine marble are:
a) Dilatation method.- This method approaches the estimation of marble thermal expansion coefficient due to either heating or cooling.
b) Ultrasonic measure of occurrence time.- allows diagnosis of stone damage due to changes in temperature.
c) Hg porosymeter- This method allows to get an insight of pore radius distribution, pore volume, porosity and the specific surface. After doing the experiments it can be estimated the change in pore volume.
d) Microfluorescense- This method can be applied on a damaged piece for detection of micro and macro fractures, as well as the pore sixe and network.
e) Tension calibration- This method measures tension response as function of temperature, usually the installation exhibits problems and when the procedure ends usually the material is lost.
f) Acoustic emission method- This procedure is based on micro seismic and micro acoustic measurements. In marble stones the mechanical stress leads to accumulation of elastic energy, then a raise in stress induces short period movements in the structure producing the acoustic emission event, if the rock is damaged then a sound of 107Hz can be registered. Use of sensors allows calculate the place where the damage exists. It is reported that new marble has small porosities while aged marble has big porosities.
Materials testing usually is done by destructive tests like uniaxial compression, triaxial deformation, as well as the drilling resistance measurement; but an alternative to test historic buildings has been found in the ultrasonic waves technique [5], since propagation is lowered if the material is weathered; results of this technique agrees with results from traditional techniques.
There is reference [6] that some archaeological pieces at Delos exhibited weathering at walls and columns, phenomenon shown as scales, granular disintegration and pitting; all of these are attributed to marine sprays and solid deposits onto marble pieces. A study was conducted using collected pieces both aged and unaltered, which were subjected to 0.2M solutions of sodium chloride. Findings provided evidence about how the NaCl presence enhances crystal formation of halite, gypsum and trona. Also at low saline concentrations and high humidity (>84%) it occurs water fixation and saline deposits dissolution.
It is known that all exposed heritage is subject of climate variations, so far total pollutant deposition is the sum of dry and wet deposition. Usually dry deposition is estimated by models, while wet deposition is related to rainfall which is easily collected and tested. Reported measured ions include: nitrate (NO3), sulfate (SO4), chloride (Cl), alkalinity (HCO3, CO3) and cations (Na, K, Mg, Ca) [7,8]. The aim of this paper is to get an insight about how rainfall anions can promote marble deterioration and a comparison of how porosity is affected once the anions penetrate into the samples.
Experimental Methodology
Unaltered samples were obtained from a marble quarry, a nucleus was extracted using a diamond drill bit, and later on this nucleus was segmented in slabs and placed in distilled water to remove impurities. After 24 hours, marble slabs were dried during 48 hours by exposure to room temperature. Marble weathering by specific anions was evaluated using 0.1 molar solutions of chlorhidric acid, sulfuric acid and calcium carbonate, plus a reference of distilled water. In Table 1 is reported the original weight of each slab, as well as initial pH and electrical conductivity (E.C.) for solutions. It must be pointed out that CaCO3 dissolution was favored by adding nitric acid that is the reason for the initial acid pH value.
Table 1: Initial slab weight and solution properties.
According to literature marble has low porosity, and its surface is hydrophobic, therefore to asses rainfall ions penetration into marble matrix, each sample was partially immersed (1/10th of its height) into a specific solution volume. By doing this it was expected to provoke solution penetration by capillary rising. At different time periods, slabs were extracted, placed onto paper towel to dry excess solution, weighted and some pictures were taken using an inverse stage microscopy. Also, each solution was monitored for pH and E.C.; and replenished with some original solution to keep the immersion height constant.
Experimental Results and Discussion
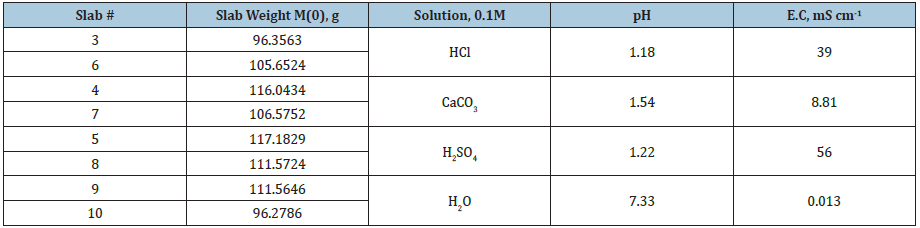
Weight evolution registers very small variations, therefore in order to visualize weight change for each slab, results are presented subtracting the original weight M(0) from the weight at time t M(t), results are reported in Figure 1, for times 4, 8, 36, 43, and 58 days.
Figure 1: Weight variations for the different slabs, in the legend AB (#) indicates the solution used and the slab number.
As it can be observed, slabs in HCl (3 and 6) exhibit an increasing rate of weight loss as time goes on, therefore it is expected an increase in porosity. Slabs placed in CaCO3 (4 and 7) are the ones with minimal weight variations, it surprises that at 36 days there was a positive raise, which drops to negative value at 43 days and the last register (58 days) shows opposite values since slab 4 increase and slab 7 decrease its weight. Otherwise, slabs into H2SO4 exhibit a weight increase up to the 36th day, after that, they lose some weight which is recovered in the following period. Slabs in distilled water H2O (9 and 10) exhibit similar trend to the ones in H2SO4 but in much lower magnitude.
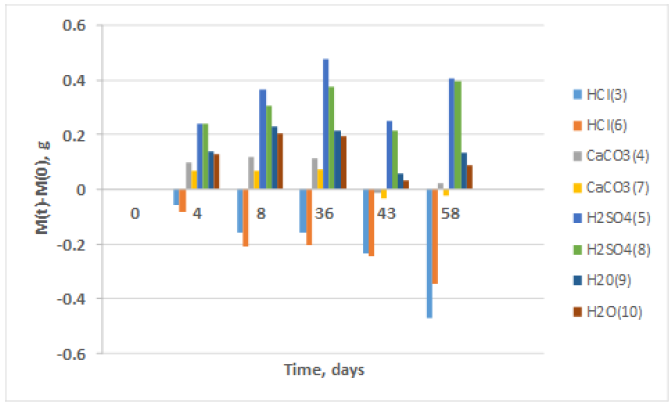
Solutions pH evolution is shown in Figure 2; in order to make evident its behavior, solution response values of the two slabs were averaged. As it can be observed at 4 days, all media have increased its pH up to values around 8, but after that time its response becomes differentiated. It is observed that HCl dropped below 6 for later on increase an keep a value around 8, CaCO3 initially reached pH about 7.5, and later on oscillates around 7, H2O exhibited a slight raise, and in the last lectures it keeps around 8. A surprising response it is observed for H2SO4 since it raises its value up to 8, drops to about 1.5 and then increased up to reaching pH near to 3, and finally it drops to 1.5.
Figure 2: pH Variation in each of the solutions.
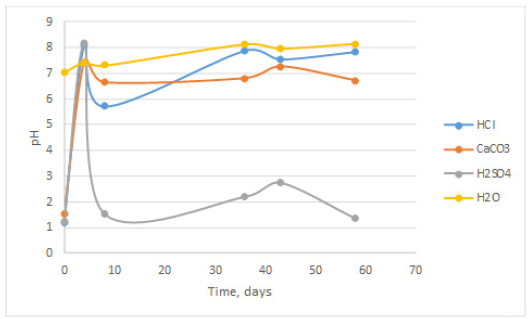
Electrical conductivity (E.C.) average values are shown in Figure 3. As it can be observed the E.C. values for H2O and CaCO3 exhibit an approximately stable behavior, HCl initially drops about 90% in conductivity, which later on increase reaching values around 10mScm- 1. Finally, H2SO4 solution exhibits a certain correlation between pH and E.C., since a raise in pH produces a drop in conductivity, while a drop in pH implies a raise in conductivity. Slab top pictures taken at the 36th day are shown in Figure 4. As it can be observed samples immersed into H2SO4 exhibit a white deposit, the others apparently do not have deposit onto them.
Figure 3: Electrical conductivity (EC) variations in each solution
Figure 4: Picture of the slab top immersed into HCl (3,6), H2SO4 (5,8), CaCO3 (4,7) and H2O (9, 10), experimental time 36 days.

Next, evidence of surface modification at each top slab is presented, pictures were taken with an inverse stage microscopy, using a 40X lens. In Figure 5 is presented the results for slab 6, immersed in HCl, as it can be observed there is a slight change in the granulometry, but there is no evidence of saline deposits. In Figure 6 it is presented the pictures for slab 7, immersed into CaCO3, similar to the HCl there is no evidence of saline deposits formation at any time.
Figure 5: Pictures of slab 6 inmersed into HCl solution at 0, 8 and 43 days.
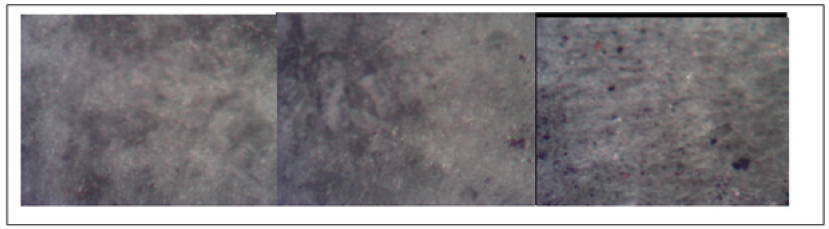
Figure 6: Pictures of slab 7 immersed into CaCO3 solution, at 0,8 and 43 days.

In Figure 7 are shown pictures of slab 8, immersed into H2SO4 solution, as it can be observed at 8 days there is a surface modification due to an incipient crystal growth, but at 43 day crystals have grown enough to be visible and easily differentiated from those present at the original surface. Chabas et al. [6] have reported pictures of the gypsum crystals, and the ones on top of slab 8 match the structure observed. In Figure 8, pictures of slab 8 at times 0, 43 and 70 days are presented, as it can be observed the last one exhibits matrix fracture, it is important to point out that surface gypsum crystals were removed in order to appreciate the slab surface.
Figure 7: Pictures of slab 8 immersed into H2SO4 at 0, 8 and 43 days.
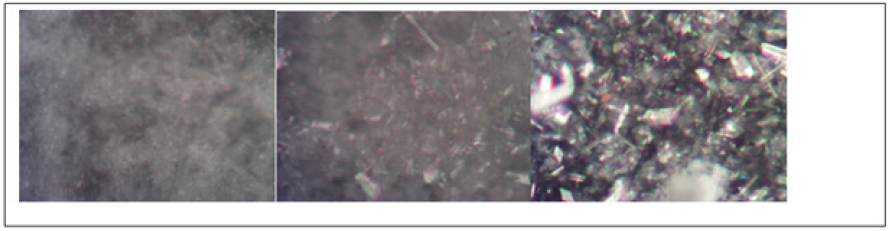
Figure 8: Pictures of slab 8 immersed into H2SO4 at 0, 43 and 70 days.

Conclusion
In this work it was presented marble weathering results due to main rain anions represented by chloride, carbonate and sulfate. Due to the initial acid pH of the solutions (HCl=1.18, H2SO4=1.22 and CaCO3= 1.54) it was expected and aggressive attack onto marble surface immersed into the solution dissolving marble and neutralizing the pH values at 4 days confirm that fact.
But later on while pH of HCl keeps close to neutrality which could imply that the CaCl2 is formed and solubilized which is the cause for weight loss and pH raise. Opposite behavior is exhibited by the H2SO4, since pH drops to acid values nearby the original one. This could be attributed to the chemical reaction
Calcite reacts forming gypsum, which is not transferred to the solution, plus the carbonic acid acidifies the solution.
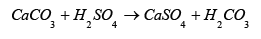
Therefore, it can be stated that both chloride and sulfate are aggressive to marble, the first produce an accelerated weight loss, while the last favor weight gain and gypsum crystals formation, which migrate to the surface producing structural damage.
References
- Abdelhafez AAM, Fatma ME, Ramadan EM, Abed-Allah AA (2012) Microbial deterioration of archaeological marble: Identification and treatment. Annals of Agricultural Science 57(2): 137-144.
- Cámara B, Mónica AB, Manuel T, Tomás FM, Mauro FLR, et al. (2017) Biodeterioration of marble in an underwater environment. Science of the Total Environment 609: 109-122.
- Eppes MC, David G (2010) Granular disintegration of marble in nature: A thermal-mechanical origin for a grus. Geomorphology 117(1-2): 170-180.
- Tschegg EK (2016) Environmental influences on damage and destruction of the structure of marble. International Journal of Rock Mechanics & Mining Sciences 89: 250-258.
- Mohamed EB, Nicólas W, Loïc M, Ronan H, Olivier R, Sébastien et al. (2015) Marble characterization by ultrasonic methods. Procedia Earth and Planetary Science 15: 249-256.
- Chabas A, Jeannette D, Lefèvre RA (2000) Crystallization and dissolution of airborne sea-salts on weathered marble in a coastal environment at Delos /Cyclades-Greece). Atmospheric Environment 34(2): 219-224.
- Báez AP, Belmont RD, García RM, Torres MCB, Padilla HG (2006) Rainwater chemical composition at two sites in central Mexico. Atmospheric Research 80(1): 67-85.
- Boumans LJM, Wattel-Kpekkoek EJ, van der Swaluw E (2014) Changes in rainwater and groundwater quality as a result of atmospheric emission reductions. Acidification and eutrophication, 1989-2010. National Institute for Public Health and the Environment (RIVM) Report 680720007/2014.
© 2020 Margarita Teutli León. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






