- Submissions

Full Text
Research in Medical & Engineering Sciences
Recent Developments of Decentralized Clinical Trial Technology in Korea
Heejin Kim1, Wooseok Yang1, Ki Young Huh2, SeungHwan Lee2, Meihua Piao3, Hyeongju Ryu4 and Kyung Hwan Kim5*
1Clinical Trials Center, Seoul National University Hospital, Korea
2Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine, Korea
3Chinese Academy of Medical Sciences, Peking Union Medical College School of Nursing, China
4Biomedical Research Institute, Seoul National University Hospital, Korea
5Department of Thoracic and Cardiovascular Surgery, Seoul National University College of Medicine, Korea
*Corresponding author: Kyung Hwan Kim, Department of Thoracic and Cardiovascular Surgery, Seoul National University College of Medicine, Seoul, 03080, Korea
Submission: May 19, 2022Published: June 01, 2022

ISSN: 2576-8816Volume9 Issue5
Abstract
Clinical trials are routinely performed on human participants to evaluate the safety and effectiveness of new treatments such as drugs and medical devices. Recent advances in Information and Communication Technology (ICT) have led to the development of Decentralized Clinical Trials (DCTs). DCTs allow participants to participate in various types of clinical trials without having to visit hospitals. Data collection and frequent interactions between participants and medical staff can be possible via remote monitoring environments. In addition, the costs and time for clinical trials conduction can be significantly reduced. The development of ICT-based DCT technologies has already initiated. However, the adoption of DCTs was facilitated by the coronavirus disease 2019 (COVID-19) pandemic. Interest in and research on DCTs has also grown in Korea. This study describes the present state of DCT technologies development in Korea.
Keywords: Decentralized Clinical Trial (DCT); Information and Communication Technology (ICT); Coronavirus Disease 2019 (COVID-19)
Abbreviations:COVID-10-Coronavirus Disease 2019; DCT-Decentralized Clinical Trial; eConsent- Electronic Consent; ICT-Information and Communication Technology; R&D- Research and Development
Introduction
Clinical trials are designed to evaluate the safety and effectiveness of newly developed medicines or medical devices in human participants. During a new drug development process, a multi-stage clinical trial is conducted, and strict procedures such as obtaining study approval are necessary at each stage [1]. Each trial should be approved by relevant regulatory authorities or ethics committees. An adequate number of well-informed participants need to be recruited and retained to achieve statistically significant results and to obtain written consent.
In conventional clinical trial settings, all procedures, including participant recruitment, data acquisition, and medical intervention, are mainly performed in hospitals. Participants have to visit hospitals repeatedly to submit their written consent and participate in the trial. Such obstacles may discourage their participation and lower retention rates. This may result in delayed or disrupted clinical trials. Another potential issue is that participants’ follow-up examinations tend to be difficult in conventional settings. To overcome these disadvantages, non-face-to-face Decentralized Clinical Trials (DCTs) based on the latest Information and Communication Technologies (ICTs) have been introduced [2,3].
Contrary to conventional centralized clinical trial settings, DCTs are normally performed in the participants’ houses or local medical institutions. The receipt of consent, data acquisition, participant monitoring, and medical intervention can be efficiently performed with a variety of mobile and wearable devices in compliance with the Clinical Trials Transformation Initiatives guidelines on mobile technologies [4]. Study safety and compliance can be enhanced with more frequent interactions between participants and medical staff through remote monitoring environments. These advantages allow participants to participate in clinical trials more easily in their daily lives. The development of ICT-based DCT technologies has already initiated. However, the adoption of DCTs was facilitated by the coronavirus disease 2019 (COVID-19) pandemic [5,6]. Interest in and research on DCT technologies has also concomitantly grown in Korea. In this study, recent developments in DCTs in Korea are presented. Figure 1 shows a basic concept of DCT.
Figure 1Basic concept of DCT.
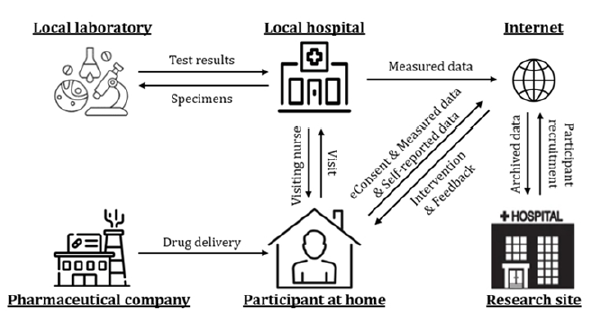
DCTs in Korea
Recent global trends in clinical trial decentralization suggest that the utilization of core DCT components, such as digital data collection, remote monitoring, electronic consent (eConsent), and logistics, has increased in high-income countries, and approximately 450 telemedicine studies have been conducted with DCT settings in 2021 [7]. The ratios of both single- and multi-country clinical trials involving DCT components have recently been higher in highincome countries than in middle-income countries. Specifically, 12.8% of single-country clinical trials in the United Kingdom were performed with DCT settings in 2019. However, simultaneously, only 1.2% of single-country clinical trials in Korea were performed with DCT settings. Moreover, Korea showed the lowest ratio of multi-country clinical trials involving DCT components (6.4%) among the surveyed countries.
For the widespread use of DCT settings in Korea, regulations should first be revised. Currently, telephone counseling, drug prescription and delivery are temporarily allowed only for COVID-19 treatment [8]. However, such services are otherwise strictly prohibited by law. Controversy regarding non-face-to-face treatment persists among stakeholders, and legal provisions such as the Personal Information Protection Act and Designation of Institutes for Clinical Trials need to be amended. However, despite such circumstances, it is encouraging that the Korea’s Ministry of Food and Drug Safety has started to establish guidelines for DCTs with pharmaceutical companies and university hospitals [9].
The development of core DCT technologies has nonetheless progressed in Korea in recent years. In 2017, the Korean government announced a five-year plan to support the pharmaceutical industry’s development. This plan includes the establishment of smart clinical trial platforms and an institutional foundation for rapid and efficient clinical trial performance. In response to this policy, there have been ongoing academic efforts to develop DCT technologies. Table 1 presents core DCT components.
Table 1Core DCT components.
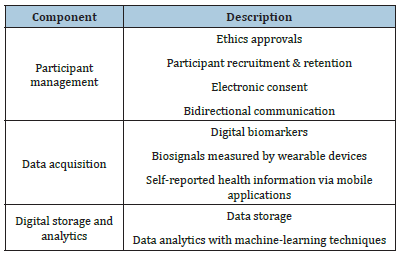
A research project was conducted to develop a next-generation smart clinical trial platform by a consortium of seven university hospitals and the Korea National Enterprise for Clinical Trials. In this project, an ICT-based clinical trial platform with a selfreporting function, automatic participant selection technology, and clinical trial management system, was developed [10,11]. A blockchain-based dynamic eConsent platform was implemented, and its performance was evaluated in a multicenter clinical trial [12,13]. Multiple clinical trial data, including consent (which used to be handwritten) can be securely stored by distributed ledger technologies while participants’ privacy is preserved [14]. This advantage can improve the transparency and reliability of the managed data. Companies are also developing further core technologies for DCTs. In the US, Medidata has provided a comprehensive DCT solution covering electronic data capturing, electronic clinical outcome assessment, and central statistical analytics [15]. Several Korean contract research organizations have adopted these solutions to establish clinical trial service platforms. Meanwhile, other companies have also been developing their own DCT technologies. JNP Medi, Korea’s first member company of the Decentralized Trials & Research Alliance, has been developing the Maven DCT Suite to release it in the first half of 2022. In the last decade, Marken Korea, a cold chain logistics company specializing in healthcare products, has provided a Direct-To-Patient service that connects clinical trial institutions and participants. Recently, this company released the DCT Home HealthCare program to enhance participants’ convenience enrolled in DCT studies.
Conclusion
ICT-based DCT technologies can be efficient for participant recruitment and clinical trial data collection and analysis. Their importance has increased during the ongoing COVID-19 pandemic. However, compared to other high-income countries, DCTs have been employed less frequently in Korea, and core DCT components are still in the early stages of research and development (R&D). Korea possesses a high-quality ICT and healthcare infrastructures. If R&D progresses and relevant regulations are eased, core DCT technologies can be improved and new comprehensive DCT platforms of excellence can be created. Thus, the costs and time for clinical trials conduction can be considerably reduced. Ultimately, Korea’s clinical trial industry will become more competitive, which promotes the development of Korea’s pharmaceutical industry.
Acknowledgement
This research was funded by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health & Welfare, Korea (grant number HI19C0790).
References
- Steardo A (2022) How to prepare a protocol for a clinical trial? 5 step advices. Pharm Methods 13(1): 11-
- Van Norman GA (2021) Decentralized clinical trials: The future of medical product development? JACC Basic Transl Sci 6(4): 384-38
- Inan O, Tenaerts P, Prindiville S, Reynolds H, Dizon D, et al. (2020) Digitizing clinical trials. NPJ Digit Med 3(1): 1-
- Herrington WG, Goldsack JC, Landray MJ (2018) Increasing the use of mobile technology-derived endpoints in clinical trials. Clin Trials 15(3): 313-31
- Xue JZ, Smietana K, Poda P, Webster K, Yang G, et al. (2020) Clinical trial recovery from COVID-19 disruption. Nat Rev Drug Discov 19(10): 662-
- AlSaleh KA (2021) Clinical trials before, during, and after COVID-19 pandemic. Am J Clin Oncol 44(2): 90-
- Hillman A, Castaneda R(2022) Who and what is at the crest of the clinical trial decentralisation wave? Clinical Trials Arena.
- Song HS, Lee B-M (2021) The viability of online pharmacies in COVID-19 era in Korea. Int J Heal Policy Manag.
- Bohe Y (2021) Discussion on the development of post-COVID-19 non-face-to-face clinical trial guidelines. Dailymedi.
- Ryu H, Piao M, Kim H, Yang W, Kim KH (2022) Development of a mobile application for smart clinical trial subject data collection and management. Applied Sciences 12(7): 3343.
- Kim H, Huh KY, Piao M, Ryu H, Yang W, et al. (2022) Self-reporting technique-based clinical-trial service platform for real-time arrhythmia detection. Applied Sciences 12(9): 4558.
- Huh KY, Moon SJ, Jeong Su, Kim MJ, Yang W, et al. (2022) Evaluation of a blockchain‐based dynamic consent platform (METORY) in a decentralized and multicenter clinical trial using virtual drugs. Clin Transl Sci 15(5): 1257-1268.
- Huh KY, Jeong S-u, Moon SJ, Kim M-J, Yang W, et al. (2022) METORY: Development of a demand-driven blockchain-based dynamic consent platform tailored for clinical trials. Frontiers in Medicine 9: 1366.
- Kuo T-T, Kim H-E, Ohno-Machado L (2017) Blockchain distributed ledger technologies for biomedical and health care applications. J Am Med Inform Assoc 24(6): 1211-
- Medidata (2022) The medidata decentralized clinical trials program.
© 2022 Kyung Hwan Kim. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






