- Submissions

Full Text
Peer Review Journal of Solar & Photoenergy Systems
Internal Shading Effect and Active Sites Number: Major Limitations in the Measuring of TOF and Quantum Yield in Heterogeneous Photocatalysis
Mohamad El-Roz*
Normandy University, France
*Corresponding author: Mohamad El-Roz, Normandie Univ, ENSICAEN, UNICAEN, CNRS, Laboratoire Catalyse et Spectrochimie, 14050 Caen, France
Submission: December 28, 2020;Published: October 20, 2021

Volume1 Issue4 October, 2021
Abstract
Operando IR spectroscopy and steady state transient isotopic exchange (SSITKA) are used to highlight the internal shading effect and surface sites heterogeneity in heterogeneous photocatalysis. Different TiO2-P25 samples are prepared in different shapes and tested under the same experimental conditions. These samples show different kinetics (SSITKA cross points) for the surface species (formate) and gas phase products during 12CH3OH/13CH3OH photo-oxidation. The difference is assigned to the shading effect and is negligible when the photocatalyst layer is lower than 10μm (for I0 = 12mW/cm2 at λirr = 366nm). Therefore, a homogeneous irradiation of the photocatalyst bed is achieved on TiO2 thin film. In the second part of this work we show that the difference between SSITKA cross points for the surface and gas phase species may also result from surface sites heterogeneity. In this work we demonstrate that the reaction rate (per gram per unit time) often used in the photocatalysis community, has no meaning on its own in heterogeneous photocatalysis under soft irradiation for a relatively thick layer (>30μm). In other hand, including the irradiated surface in the calculation of the reaction rate seems more representative than the mass of the photocatalyst, for comparing efficiencies of heterogeneous photocatalysts for a given reaction.
Keywords:Photocatalysis, SSITKA, TOF, FTIR-Operando, TiO2
Introduction
Heterogeneous photocatalysis has become an important and promising approach for durable solutions for environment [1-3], renewable energy [4-6], and green chemistry [5,7]. Wide and serious applications of photocatalysis in these fields are mainly based on the design of new materials with high performance. Significant work has been performed to improve photocatalytic systems, particularly those operating under visible light, based on either solid oxides or non-oxides. However, quantitative comparison of these systems and advances in fundamental understanding of the photocatalysis reaction are, unfortunately, very challenging due to the absence of standardization in both the measurement and the recording of heterogeneous photocatalysis activity data [8]. The development of common measurement protocols is definitely critical for advancing and expanding the field of heterogeneous photocatalysis.
Turnover frequency (TOF) and quantum yield (QY) are often used as standard parameters
in thermal catalysis and photochemistry [9-11]. However, there are specific problems for their
use in heterogeneous photocatalysis [12,13]:
A. TOF is defined as the number of molecules converted per active site per time unit.
Indeed, the number of active sites is a very limited value for most systems, as not only
the definition of what an active site is, but also the number of active sites are ill-defined.
B. QY is defined as the number of molecules undergoing an event (conversion of reactants
or formation of products) relative to the number of energy quanta absorbed by the
reactant(s) or by the photocatalyst. The number of photons absorbed by heterogeneous
photocatalysts is experimentally difficult to access, owing to reflection, scattering and
internal shading effect.
Consequently, the QY values are frequently rough approximates. The term “apparent quantum yield” (AQY) is probably used too often in heterogeneous photocatalysis. It is defined as the relative number of molecules converted to the total number of “incident” photons on the reactor walls, for sometimes ill-defined reactor geometry and for a large spectral irradiation window (polychromatic radiation).
In this work, operando-IR spectroscopy is used to demonstrate how the shading effect and the sites heterogeneity on the photocatalyst surface can alter the TOF and QY values determined in heterogeneous photocatalysis. For this purpose, several experiments of methanol photooxidation on TiO2 photocatalysts were carried out under the same experimental conditions of irradiation, temperature, flow and reactor geometry, while changing the sample geometry. Different Steady State Isotopic Transient Kinetic Analyzes (SSITKA) experiments have been performed. These experiments provide a correlation between the surface species and the gas phase products showing how the shape and the nature of the photocatalysts can lead to wrong conclusions. This work is a strong basis for a future determination of TOF in heterogeneous photocatalysis using operando IR spectroscopy with the SSITKA approach.
Experimental Section
The photocatalytic oxidation of methanol was performed using the operando IR reactor described elsewhere [14]. Hierarchical TiO2 (TiO2-L) has been synthesized and characterized as described by [15]. The seeding of natural Luffa sponge (used as a biotemplate) with anatase TiO2 was accomplished by a 24h hydrothermal synthesis at 373K, under autogeneous pressure. A synthesis mixture with the following composition 10Ti(OEt)4sub>:45C2H5OH:45H2O (vol%) was used. The pre-cursor solution was stirred with Luffa sponge at room temperature (RT) for 1h. Then, 100mL of the solution (together with 500mg of Luffa sponge) was poured into Teflon-lined stainless-steel autoclaves. After the synthesis, the Luffa sponges were removed from the autoclaves, thoroughly cleaned (in order to remove the excess of TiO2 seeds loosely bound on the substrate) with ethanol and water and dried overnight at 323K. After rinsing, the composite (TiO2/Luffa) was calcined under air, with a 5h ramp to 773K and a 5h dwell time.
A macrotubular TiO2 shape with macrochannels was obtained after removal of the natural organic support (replica of Luffa fibers). Then, TiO2-L samples were characterized using X-ray diffraction (XRD), N2 sorption, and ultraviolet-visible light (UVvis) spectroscopy [16]. As a comparison, photooxidation was also performed on a commercial TiO2 from Evonik-Degussa (TiO2-P25). Photocatalysts were pressed into self-supported wafers (Ø = 16mm, m = 10mg/cm2; thickness = 60μm (measured by Micromaster-IP54 and SEM)) or deposited as thin film (<10μm) on a KBr window (for a SSITKA experiment). The IR reactor-cell was connected to gas lines with gas mixing devices and mass flow controllers. Two gas mixtures can be prepared and sent independently to the reactor cell. Exhaust gases (reactants and/or reaction products) can be analyzed by a Quadrupole Mass Spectrometer (Pfeiffer Omnistar GSD 301), while complementary information on the gas phase can be gained by IR spectroscopy with a gas micro-cell.
IR spectra (64 scans per spectrum) of the catalyst under working conditions were collected at a time resolution of 1 spectrum each 2 minutes with a Thermo Scientific Nicolet 6700 spectrometer, equipped with a MCT detector. A greater temporal resolution has been used for SSITKA experiments: 10 scans per spectrum and 1 spectrum collected each 5.4 seconds. All IR spectra are displayed as absorbance. For this specific study, the system was equipped with two saturators in the same thermostatic bath in order to obtain similar temperatures. A fixed concentration of vaporized methanol, either in its natural form or 99.0% 13CH3OH enriched (Cambridge Isotope Laboratories) was sent, via a two ways valve, under the flow conditions: 1200ppm methanol, 20% oxygen in Argon at 60,000h-1 constant gas hourly space velocity.
In order to calculate the methanol conversion, a calibration curve was drawn to establish the linear relationship between the concentration of methanol and the MS signal intensity or IR band intensity (at m/z = 29 and 1038-1026cm-1 respectively), for 12CH316OH. The reaction was studied at 301K. The irradiation was applied with a Xe-Hg lamp (LC8 Spot Light Hamamatsu, L10852, 200W) and UV-light guide (A10014-50-0110) mounted at the entrance of the operando IR cell. Details of the setup were previously reported [16]. A monochromatic 365nm band pass filter was used. The variation of the irradiated surface has been carried out using metallic masks with different opening diameters. The photocatalyst samples were activated at 473K (5K/min) for 1h under synthetic air and polychromatic UV irradiation and then cooled down to 301K (5K/min) before each experiment. The characteristic IR bands used to quantify the gas phase and adsorbed species before and during natural and labeled methanol photooxidation reactions by FTIR were taken from our previous work [17]. The thickness of the photocatalyst pellets/layer has been determined using scanning electron microscopy (SEM).
Result and Discussion
Internal shading effect in heterogeneous photocatalysis
In order to highlight the shading effect of TiO2 nanoparticles, different SSITKA experiments were performed on different shapes of TiO2-P25 photocatalyst. SSITKA is a methodology for obtaining transient conditions while remaining under the required chemical and/or kinetic steady state environment for a given reaction. It has already been used to elucidate the mechanism of a catalytic reaction, and sometimes to determine some thermodynamic parameters such as the activation energy [16,18-20].
Here, SSITKA studies of 12CH3OH/13CH3OH were performed on three TiO2-P25 samples under the same experimental conditions of temperature (301K), irradiation (I0 ≈ 15mW/cm2; λ= 366nm) and gas flow (20cm3/min). The photocatalyst surfaces were monitored by transmission FTIR. After UV irradiation of TiO2-P25 pellet saturated with 12CH3OH, new bands appeared, assigned to formate species adsorbed on the catalyst surface (Figure 1). The bands located at 1571, 1378, 1362, 1110, and 1023cm-1 are assigned to nas(COO), δ(CH), ns(COO) of formate species, ν(OC) (linear) of methoxy and methanol physisorbed, respectively [17]. A shift of these bands to lower wavenumbers (except for δ(CH)) was observed when 13CH3OH was used. The kinetic analysis of the formate and methoxy 12C/13C-exchanges were performed using the integration of the nas(COO) and ν(OC) bands of formate and methoxy species, respectively. The gas phase products were analyzed with on line MS and gas-FTIR.
Figure 1:IR spectra of TiO2 pellet (10mg/cm2) during the photooxidation of 12CH3OH and 13CH3OH (methanol concentration = 1200ppm, flow = 20 cm3/min; 301K; λ = 366nm; I0 = 15mW/cm2).

Starting from the chemical steady state (t = 0), the reaction flow was switched from natural to 13C-labeled methanol. Consequently, the bands of adsorbed 13C-methoxy (1088cm-1) and 13C-formates (1342 and 1528cm-1) species progressively replaced those of the unlabeled species (Figure S1), giving rise to several isosbestic points. Figure 2 shows the time evolution of the adsorbed species on TiO2-P25 samples studied under different shapes: a) self-supported pellet (20mg, 16mm of diameter and 60mm of thickness); b) TiO2 supported on the center of a 16mm stainless steel grid (sample diameter: 6mm; 65mm of thickness); c) thin film of TiO2 powder deposited on a KBr window (16mm of diameter; <10mm of thickness). The methanol concentrations were adapted to the mass of the photocatalyst used (1200; 500 and 500ppm, respectively), to work at a constant contact time and in order to obtain a similar selectivity. The evolution of the gas phase products was monitored using the characteristic m/z signals of the products (29/33 for 12CH3OH/13CH3OH; 44/45 for 12CO2/13CO2; and 60/62 for 12CH312CHO/13CH313CHO).
Figure S1:IR spectra of TiO2-P25 during a SSITKA experiment for which an initial flow of 1200ppm of 12CH3OH, 20% of oxygen diluted in Ar (total flow=20cm3sup> min-1sup>) was switched to a similar but labeled (13CH3OH) flow (the spectrum recorded at t = 0 was used as background).
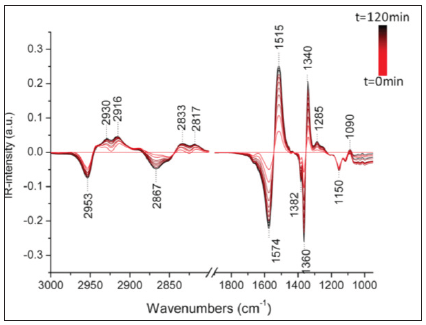
Figure 2: Evolution of adsorbed species on TiO2 surface and in gas phase during methanol photooxidation
versus time of the 12CH3OH/13CH3OH SSITKA experiment (t=0 min corresponds to the 13C

Figure 2 shows that similar crossing points were observed at t ≈ 3.0±0.3min and t ≈ 16±2min for adsorbed methoxy and formate species, respectively, when TiO2-P25 was tested as a self-supported pellet or as deposited powders on a stainless steel grid support. The online analyses of the exhaust gases showed similar crossing points (at t ≈ 3.7±0.7min) for methanol, carbon dioxide, and methylformate. Methoxy species were thus in good correlation with gas products. This was not the case with formate species. Only a small amount of the formate species were involved in methanol photooxidation. These results may lead to a very confusing conclusion, that formate species are not the main intermediate.
Figure 3:
Infrared (IR) spectra of (A) TiO2-L and (B) TiO2-P25 after 60min of UV irradiation during methanol photooxidation. (Conditions: 1200ppm of methanol in synthetic air; flow = 25cm3/min; T = 301K; I0(366) ≈ 15mW/cm2; λ = 366nm)

In a recent work, we demonstrated that formate species are intermediates in the two photooxidation reactions of methanol into carbon dioxide and into methylformate [17]. Two possibilities may explain such apparent contradiction: i) the shading effect of TiO2 particles and the photocatalyst site’s activity depending on the location of the sites, either near the wafer surface or in the bulk (inhomogeneous irradiation with the depth of the catalyst in the catalyst bed) and/or ii) the presence on TiO2 surface of different surface sites with different activity Figure 3. A SSITKA experiment on a thin film of TiO2-P25 (thickness <10mm, deposited on a KBr window) has been performed to exclude or not the internal shading. In that case, a good correlation between the formate species and gas phase products was observed with similar crossing points at around 0.8±0.2min. This confirms the internal shading as one of the most important obstacles to mechanism description and QY determination.
Further work should be performed in order to validate a possible quantitative relationship between the time to the crossing points of the 12C/13C of formates and the pellet thickness of TiO2-P25 or the irradiation intensity. The approach used here is also very promising for determination of TOF in heterogeneous photocatalysis under ideal conditions (homogeneous irradiation of the photocatalyst bed). To reach this goal, the relationship between isotopic exchange kinetics and TOF must be established with a quantification of the real active sites.
Surface sites heterogeneity of photocatalysts
The number of accessible active sites is the most important parameter for calculating TOF values in heterogeneous photocatalysis. Here, the heterogeneity of the photocatalyst surface sites is the limiting step. In such a case, the traditional methods for the determination of active sites, such as reactive chemisorption, are no more possible. Spectroscopic analysis of the photocatalyst surfaces during the reaction (under operando condition) is very useful, giving information about the activity of the surface sites.
In this part, the heterogeneity of site activities on photocatalyst surfaces is highlighted using the same methodology (operando-IR & SSITKA). Two different TiO2 photocatalysts (TiO2-P25 and TiO2-L) were tested under similar conditions. The photocatalysts were prepared as thin pellet (60mm) in order to obtain a high precision on their thickness and mass. The internal shading was, therefore, similar and did not preclude the comparison between both photocatalysts.
The characteristics of TiO2 photocatalysts and their activity in methanol photooxidation are summarized in Table 1. Despite a higher surface area for TiO2-L in comparison with that of TiO2-P25 (1.6 times higher), the methanol conversion rate was 1.5 times lower, with a CO2-selectivity 1.4 times higher.
Table 1:
Characteristics of TiO2-L and TiO2-P25 Photocatalysts used in this Work and their photocatalytic activity in the photooxidation of 1200ppm of methanol (conditions: TiO2 pellet (∼20mg; thickness ∼ 60mm; 20% O2/Ar vol %; flow = 25cm3/ min; T= 301K; I0(366) ∼10mW/cm2; λ = 366nm).

Figure S2: Evolution CO2 (A) and methylformate (B) in gas phase during methanol photooxidation on TiO2-P25 and TiO2-L, versus time of the 12CH3OH/13CH3OH SSITKA experiment (t=0min correspond to the 13CH3OH/CH3OHexchange). (Conditions: 1200ppm of methanol in synthetic air; flow = 25cm3/min; T=301K; I0(366) ≈ 10mW/cm2; λ = 366nm.)

Operando IR in the photocatalytic reactor shed more light on these results. Different vibration bands for bidentate formates on TiO2-L (maxima at 1600, 1373, and 1356cm-1) and on TiO2-P25 (maxima at 1570, 1379, 1362cm-1) were observed, probably because of a difference in the nature of the adsorption sites. In addition, the IR intensity of these bands was much higher on TiO2-L than on TiO2-P25. This may be explained by the higher specific surface of TiO2-L, leading to a greater formation of formate intermediates. In such a case, the methanol conversion rate would have been higher for TiO2-L, which was not the case. Hence, this difference was probably due to a stronger adsorption of bidentate formate species on TiO2-L than on TiO2-P25, thus reducing their transformation rate (Figure S2).
In the SSITKA experiments using operando FTIR, the crossing point for the exchange of formates on TiO2-L was observed 60 minutes later than on TiO2-P25 (Figure 4). The same kinetics for the isotopic exchanges in the gas phase products was observed, with a similar crossing point for both photocatalysts, at around 3±0.3 minutes (Figure S3). In addition to the previous results, this experiment shows that an important part of formate species is more stable on TiO2-L surface than on TiO2-P25
Figure 4:
Evolution of formate species on TiO2-P25 and TiO2-L surfaces during methanol photooxidation versus time of the 12CH3OH/13CH3OH SSITKA experiment (t=0 min corresponds to the 13CH3OH/CH3OH exchange). (Conditions: 1200ppm of methanol in synthetic air; flow = 25cm3/min; T = 301K; I0(366) ≈ 15 mW/cm2; λ = 366nm). (Doted lines correspond to the trend lines of the formate evolution)
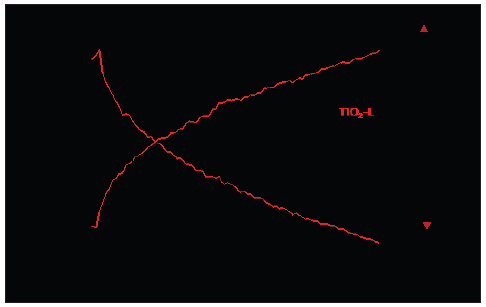
Figure S3:
(A) Evolution of the IR band of TiOH at 3690cm-1 and of formate species at 1602cm-1 during methanol photo-oxidation. (B) Evolution of the formate species IR-band vs Ti (III) OH IR-band during methanol photo-oxidation.
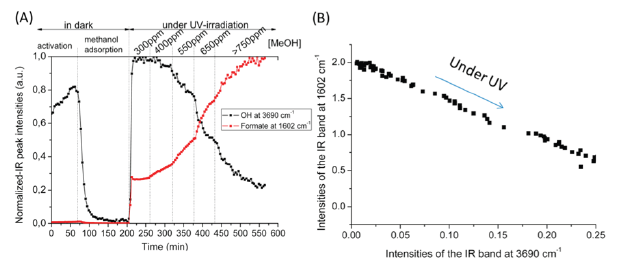
On the other hand, a good relationship was observed between the TiOH hydroxyl groups (stretches at 3690cm-1), and formate species: the increase of the IR band intensities was accompanied by a decrease of the IR band intensities for TiOH groups (at 3690cm-1, Figure S3). These results seem to indicate that formate intermediates are stabilized on the sites previously occupied by the hydroxyls, therefore corresponding to coordinatively unsaturated surface sites. Therefore, the determination of the number of actives sites should take into account the presence of such sites with no or low activity.
Variation of the photocatalyst’s efficiency versus their mass under soft irradiance
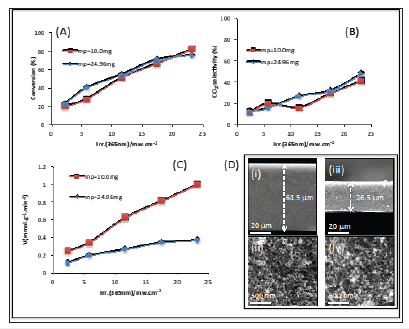
Self-supported catalysts with relatively thick pellets (>30mm) may have constant efficiency whatever the sample mass when the irradiation is too soft (intensity<50mW/cm2). Figure 3 illustrates the variation of the photocatalyst efficiency and the reaction selectivity of methanol photooxidation versus irradiation intensity for two self supported TiO2-P25 with different mass and pellet thickness (Figure 3D). The results demonstrate an increase of efficiency with increasing light intensity, still in a reasonable proportion with a monochromatic light in comparison with a polychromatic irradiation. However, still no effect of the photocatalyst mass/thickness is observed. This is due mainly to the shading effect highlighted in the previous part (same light penetration depth). Therefore, the calculation of the reaction rate per unit of photocatalyst mass can lead to confusing results: comparing two different photocatalysts with different masses can produce an under-estimation of the photocatalytic activity of one sample due to the shading effect (Figure 3C). The reaction rate, per gram per unit time, has no meaning on its own in heterogeneous photocatalysis (mainly measured in the gas phase for self supported catalyst) under soft irradiation for a relatively thick layer (>30mm). In other words, in the previously cited conditions, it is better not to express results versus the mass of the photocatalyst.
Photocatalyst efficiency vs irradiated surface: A possible solution
Whereas the mass plays no major effect under soft irradiation conditions, the irradiated surface is a very important factor for estimating the photocatalyst activity. Figure 4 demonstrates how increasing the irradiated surface of the pellet increases methanol conversion [18-21]. On the other hand, the selectivity is not significantly affected. Therefore, including the irradiated surface in the calculation of the reaction rate seems very important, even more than the mass of photocatalyst, for comparing efficiencies of heterogeneous photocatalysts for a given reaction Figure 5.
Figure 5:
Evolution versus the intensity of the monochromatic irradiation at 365nm of methanol conversion (A) and CO2-selectivity of the reaction (B) Over self supported TiO2-25 pellets prepared with two different mass of photocatalyst. (C) Shows the evolution of the reaction rate (in mmol.g-1.min-1). (D) SEM images of side (i/iii) and top (ii/iv) views of self supported TiO2-P25 pellet prepared with 24.96 (i/ii) and 10.00 (iii/iv) mg of powders. Condition of the photocatalytique tests: l=365nm; methanol concentration= 1200ppm; 20% O2/Ar, flow= 25cm3.min-1.
Conclusion
Operando IR coupled to SSITKA is a very promising approach for a fundamental understanding of the photocatalytic reaction. In this work, we have used this technique to investigate the internal shading effect and surface sites heterogeneity in heterogeneous photocatalysis, as key factor parameters for QY and TOF determination Figure 6. We have shown that calculation of TOF is only possible after optimizing irradiation conditions (homogeneous irradiation of the photocatalysts bed) and identifying/quantifying the active sites. We also demonstrate that normalizing the reaction rate to the photocatalyst mass can lead to wrong conclusions on the efficiency of a system. Our advice is to use the irradiated surface of the photocatalyst when relatively thick layers (>30mm) of selfsupported heterogeneous photocatalysts are used under relatively soft irradiations conditions.
Figure 6:
Evolution of the conversion (in %) and CO2-selectivity of methanol photooxidation on TiO2 P25 pellet (m=25mg) versus the irradiated surface. Desired irradiated surface is obtained by using different masks. Condition: l=365nm; irradiance= 17.5mW/cm2. methanol concentration = 1200ppm; 20%O2/Ar, flow= 25cm3.min-1.
p>
References
- El Roz M, Monika K, Cool P, Thibault SF (2012) New Operando IR Technique to Study the Photocatalytic Activity and Selectivity of TiO2 Nanotubes in Air Purification: Influence of Temperature, UV Intensity, and VOC Concentration. J Phys Chem C 116(24): 13252-13263.
- Alonso TA, Robert D, Keller V, Keller N (2014) H2S photocatalytic oxidation over WO3/TiO2 Hombikat UV100. Environ Sci Pollut Res 21(5): 3503-3514.
- Pelaez M, Nolan NT, Pillai SC, Seery MK, Falaras P, et al. (2012) A review on the visible light active titanium dioxide photocatalysts for environmental applications. App Catal B: Environ 125: 331-349.
- Singh V, Beltran IJC, Ribot J C, Nagpal P (2014) Photocatalysis Deconstructed: Design of a New Selective Catalyst for Artificial Photosynthesis. NanoLett 14(2): 597-603.
- Doherty R (2014) Photocatalysis water is the solution. Nature Chem. 6, 168-169.
- Zhang M, El Roz M, Frei H, Mendoz CJL, Head GM, et al. (2015) Visible Light Sensitized CO2 Activation by the Tetraaza [CoIIN4H(MeCN)]2+ Complex Investigated by FT-IR Spectroscopy and DFT Calculations. J Phys Chem C 119(9): 4645-4654.
- Herrmann JM, Duchamp C, Karkmaz M, Hoai BT, Lachheb H, et al. (2007) Environmental green chemistry as defined by photocatalysis. J Hazard Mater 146(3): 624-629.
- Wachs IE, Phivilay SP, Roberts CA (2013) Reporting of Reactivity for Heterogeneous Photocatalysis. ACS Catal 3(11): 2606-2611.
- Bandara J, Morrison C, Kiwi j, Pulgarin C, Peringer P (1996) Degradation/decoloration of concentrated solutions of Orange II. Kinetics and quantum yield for sunlight induced reactions via Fenton type reagents J. Photochem Photobio A: Chem 99: 57-66.
- Serpone N, Salinaro A, Emeline A, Ryabchuk V (2000) Turnovers and photocatalysis: A mathematical description. J Photochem Photobio A: Chem 130(2-3): 83-94.
- Troppmann S, Kçnig B (2014) Functionalized Vesicles with Co-Embedded CdSe Quantum Dots and [FeFe]-Hydrogenase Mimic for Light-Driven Hydrogen Production. Chem Eur J 20: 14570-14574.
- Serpone N, Terzian R, Lawless D, Kennepohl P, Sauvé G (1993) On the usage of turnover numbers and quantum yields in heterogeneous photocatalysis. J Photochem Photobio A: Chem 73(1): 11-16.
- Kisch H (2010) On the problem of comparing rates or apparent quantum yields in Heterogeneous Photocatalysis. Angew Chem Int Ed 49(50): 9588-9589.
- El Roz M, Bazin P, Daturi M, Thibault SF (2013) ACS Catal. Operando Infrared (IR) Coupled to Steady-State Isotopic Transient Kinetic Analysis (SSITKA) for Photocatalysis: Reactivity and Mechanistic Studies 3(12): 2790-2798.
- El Roz M, Bazin P, Thibault SF (2013) An operando-IR study of photocatalytic reaction of methanol on new *BEA supported TiO2 Catal Today 205: 111-119.
- El Roz M, Haidar Z, Al Lakiss L, Toufaily J, Thibault SF (2013) RCS Adv 3: 3438-3445.
- El Roz M, Bazin P, Daturi M, Thibault Starzyk F (2015) On the mechanism of methanol photooxidation to methylformate and carbon dioxide on TiO2: an operando-FTIR study. Phys Chem Chem Phys 17: 11277-11283.
- Efstathiou A M, Verykios X E (1997) Transient methods in heterogeneous catalysis: Experimental features and application to study mechanistic aspects of the CH4/O2 (OCM), NH3/O2 and NO/He reactions. Applied Catalysis A: General 151(1): 109-166.
- Olympiou GG, Kalamaras CM, Zeinalipour YCD, Efstathiou AM (2007) Mechanistic aspects of the water-gas shift reaction on alumina-supported noble metal catalysts: In situ DRIFTS and SSITKA-mass spectrometry studies. Catal Today 127(1-4): 304-318.
- Bazin P, Thomas S, Marie O, Daturi M (2012) New insights into the methanol oxidation mechanism over Au/CeO2 catalyst through complementary kinetic and FTIR operando SSITKA approaches. Catal Today 182: 3-11.
- Yang XJ, Chen B, Zheng LQ, Wu LZ, Tung CH (2014) Highly efficient and selective photocatalytic hydrogenation of functionalized nitrobenzenes. Green Chem 16(3): 1082-1086.
© 2021 Mohamad El-Roz. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)













.bmp)
.jpg)
.png)
.jpg)














.png)

.png)



.png)






