- Submissions

Full Text
Trends in Telemedicine & E-health
Telemonitoring in Chronic Lung Disease: From Parameters and Tools to an Ideal Organizational Model
Giovanna Panarello1,2, Paolo Capuano1,2*, Gennaro Martucci1,2, Stefano Tigano3, Nicolò Rizzitello1, Giuseppe Raffa1,4, Lavinia Martino1,5, Nicola Lanzarone1,5, Patrizio Vitulo1,5, Alessandro Bertani1,5, Antonio Arcadipane1,2 and Diego Bellavia6
1University of Pittsburgh Medical Center (UPMC), Italy
2Department of Anesthesia and Intensive Care, IRCCS-ISMETT (Mediterranean Institute for Transplants and Highly Specialized Therapies), Italy
3Department of Anesthesia and Intensive Care, AOU Policlinico-San Marco, Italy
4Department for the Treatment and Study of Cardiothoracic Diseases and Cardiothoracic Transplantation, IRCCS-ISMETT, Italy
5Thoracic Center, IRCCS-ISMETT (Mediterranean Institute for Transplants and Highly Specialized Therapies), Italy
6Research Department IRCCS-ISMETT, Italy
*Corresponding author:Paolo Capuano, Department of Anesthesia and Intensive Care, University of Pittsburgh Medical Center (UPMC), Italy
Submission: November 16, 2023; Published: December 14, 2023

ISSN: 2689-2707 Volume 4 Issue 5
Abstract
Home-based care of individuals with Advanced Lung Disease (ALD), delivered with home-monitoring tools or broader Telemedicine (TM) interventions, has the potential to reduce the inequality of access to specialist centers, reduce the health care burden, improve patients’ quality of life, and prevent adverse outcomes associated with acute exacerbations through early identification. Current literature reports a high variability in the ways TM is provided and its efficacy, therefore there is an urgent need to perform new studies to test the most effective and re-source-sparing TM organizational and practical model. The “ideal” TM approach for patients with ALD would involve a comprehensive and integrated system that addresses the specific needs of these patients. The aim of the present research proposal is to analyze the effect of a telemonitoring pilot project on direct medical costs, health resource utilization, and mortality. We will carry out a monocentric, assessor-blind, two-arm (intervention/control) randomized controlled trial, enrolling 20 patients diagnosed with ALD evaluated for lung transplantation. In the inter-vention group, home telemonitoring will be based on few simple vital parameters, assessed au-tomatically and continuously by an Artificial Intelligent System, able to detect a worrisome trend and alert the clinical team. Primary outcomes will be health care utilization, mortality, and medication use. Secondary outcomes will be health-related QoL, psychological morbidity, lung function, and cost-effectiveness..
Keywords:Telemedicine; Lung disease; Digital medicine; COPD; Outcomes
Introduction
Global impact of advanced chronic respiratory diseases on health system
According to a report from the National Centers for Disease Control and Prevention, 90% of the 3.8 trillion USD annual health care expenditure in the United States comes from pa-tients with chronic and mental illnesses [1]. Given the aging population worldwide, the management of chronic diseases is bound to become a global health challenge and an economic burden in the foreseeable future [2]. Advanced Lung Diseases (ALDs) are a heterogeneous group of disorders principally affecting the lung parenchyma, resulting in dyspnea, and often leading to respiratory failure and premature death. Though ALD epidemiology varies globally, its incidence is increasing. Management at expert ALD centers is associated with better survival, easier access to specialized drugs, and a higher likelihood of lung transplantation. However, many pa-tients, especially frail adults, might have limited access to these specialized centers be-cause of geographical distance.
For example, Chronic Obstructive Pulmonary Disease (COPD) causes elevated mortality and morbidity, as well as soaring health care expenditure and recourse to health care facilities [3,4]. COPD is expected to become the world’s fourth leading cause of death within the next decade [5]. In search of cost-effective concepts of chronic care management, researchers and policy-makers have increasingly recognized the potential of Telemedicine (TM) in reducing morbidity, mortality, and health care utilization and its associated costs [3-9] . However, current literature reports a high variability in the ways TM is provided and its efficacy, therefore there is an urgent need to perform new studies to test the most effective and resource-sparing TM organizational and practical model [10].
Current models of health care, which are typically predicated on intermittent follow-up of patients at pre-scheduled time intervals, are poorly suited to the identification and prompt treatment of acute (on chronic) events. Furthermore, the increased incidence and prevalence of ALD means that the current capacity of specialty clinics might become insufficient to serve the needs of the growing number of patients with complex care requirements. Home-based care of individuals with ALD has the potential to reduce the inequality of access to specialist centers, reduce the health care burden, improve patients’ quality of life, and prevent adverse outcomes associated with acute exacerbations through early identification. Home-based care can be delivered with homemonitoring tools or broader TM interventions. In this research proposal, we focus predominantly on home monitoring, but also briefly touch on TM interventions. The effective implementation of home-monitoring strategies for the management of patients with ALD requires several factors to be addressed: the safety of the systems and the organizational model of surveillance and care used, patient selection, and adaptability to a new model of care. These include a clear understanding of the strengths and weaknesses of digital health care devices, effective implementation of Web-based portals to facilitate secure contact and sharing of data between patients and health-care providers, and appropriate algorithms and proto-cols for ensuring that home-generated data are effectively monitored and used for patient care. Medication adherence in patients with ADL is very low and is associated with worse clinical outcomes and greater economic burden on the community. It is therefore a priority to find new ways to support such patients and ameliorate their adherence to defined medication strategy.
Importance of precision health, personalized medicine, and telemonitoring
TM refers to the use of information and communication technologies to improve patient outcomes by increasing access to care and medical information. Home monitoring is a subcategory of TM, focusing on the use of technologies that enable domiciliary collection of health status data by patients. Telemonitoring is a frequently used tool in the long-term management of many chronic diseases, such as COPD or chronic heart failure. TM approaches could be used in various ways in pulmonology: Tele-diagnostic, tele-consultation, tele-therapy, and telemonitoring of patients being treated with positive pressure devices [11]. However, conflicting data were found detailing the efficacy of TM services for managing COPD and generally pa-tients affected with ALD [10].
Home monitoring also has the potential to verify, in real time, treatment response and to identify related adverse events, thus facilitating treatment modification. Different care programs have been developed to support patients with ALD throughout their disease course, including home visits, nurse-led telephone counselling, and support for medication management. A multicenter, Randomized Controlled Trial (RCT) of a comprehensive online home monitoring program for interstitial pulmonary fibrosis, which included home spirometry, reporting of symptoms, healthrelated quality of life, drug-related side-effects, a medication coach, and e-consultations, showed a clinically significant improvement in psychological wellbeing, as measured by the King’s Brief ILD Questionnaire in the intervention group. Home monitoring was greatly appreciated by patients, and al-lowed for individually tailored medication adjustments.
Furthermore, though there are several benefits and
opportunities offered by implementing TM and home monitoring in
patients with ALD (Figure 1), several barriers and potential issues
have been brought to the attention of the scientific and medical
communities:
a. Requirement of internet access
b. Side-effects (e.g., induction of coughing during use of
home spirometry)
c. Burden of monitoring
d. Research priorities for home monitoring in ALD
e. Difficulty in handling devices for some patients
f. Results that might cause anxiety or raise questions
g. Quality control measurements (not always optimal)
h. Delayed medical contact due to false sense of security
i. Wrong medical decision-making due to inappropriate
results.
Precision health is defined as a holistic approach to helping people stay healthy through personalized prevention and treatment, which focuses on the prevention of disease. This includes precision medicine, but with a greater emphasis on daily monitoring, health promotion, and disease prevention [12]. Several studies have demonstrated the great potential of advancements in precision health to reshape human health and improve the treatment outcomes of breast, lung, and colorectal cancer [13] by providing daily critical data to reduce mortality in patients of all ages and both sexes who are suffering from the current epidemic of chronic diseases related to lifestyle habits [14,15].
A number of chronic disease prediction models have been developed in recent years. Goto et al. proposed a model to predict Acute Exacerbations of Chronic Obstructive Pulmonary Disease (AECOPD) using demographic features, vital signs, and electronic medical records in the Emergency Department (ED) [16]. They found that the use of machine learning improves the ability to predict critical care and hospitalization rates for emergency patients with COPD exacerbation over the traditional statistical approach with emergency severity index information. Likewise, Peng et al. developed a machine learning approach to predict the prognosis of AECOPD hospitalized patients with clinical indicators. They used vital signs, medical history, inflammatory indicators, and decision trees to help respiratory physicians assess the severity of the patient early and improve patient prognosis [17,18]. Home telemonitoring, based solely on verbal consult by the care team alone and combined with pre-defined action plans but with a single short educational component and without a comprehensive selfmanagement program, reduces in-hospital health care utilization and increases treatment of COPD exacerbations with corticosteroids and antibiotics [19] and is accepted favorably by patients. However, use of ALD action plans in this context is unlikely to increase or decrease mortality.
Moreover, despite a growing body of favorable evidence for TM in the management of COPD and other chronic diseases, such as Congestive Heart Failure (CHF), the benefit of telemonitoring with regard to clinical and economic outcomes has yet to be clearly demonstrated [6,7]. Meta-analyses indicate that telemonitoring reduces the odds ratio of all-cause hospitalization and ED visits by up to 54% [20-22] and 73 % [21,22], respectively, but has no impact on hospital length of stay, disease-specific quality of Life (QoL) or mortality [20-23].
However, the few telemonitoring interventions evaluated were highly heterogeneous, employing various technologies that included simple telephone calls, patient education, virtual videoconsultations, semi-automated transmission of vital parameters, or a combination of those [21]. The breadth and frequency of parameter measurements, as well as availability and qualification of support staff diverged across studies. Studies were typically under-powered [7] due to small sample sizes (range 18 (22) to 256 (23), median 70 (23)). Moreover, most studies were controlled trials and thus conducted in a well-ordered clinical environment that might lack comparability to routine care settings [20-24].
The ideal organizational model
The “ideal” TM approach for patients with ALD would involve
a comprehensive and integrated system that addresses the specific
needs of these patients. Here are some key components of an ideal
telemedicine approach:
Remote patient monitoring: The telemedicine system
should include wireless systems and devices that allow for remote
monitoring of patients’ vital signs, functional status, and disease
progression. This could include devices such as wearable sensors,
mobile apps, or home monitoring equipment to track parameters
like blood pressure, heart rate, saturation and respiration rate, and
functional reserve.
Functional evaluation at home: The TM approach should
incorporate functional evaluation tools that can be used at home.
This could involve digital platforms or applications that guide
patients through functional assessments and provide feedback on
their performance. These evaluations can help assess the patient’s
functional reserve and identify any changes that may indicate a
need for medical intervention.
Easy-to-use platforms: The TM system should have userfriendly
platforms and interfaces for both patients and caregivers.
Patients should be able to easily access and use the telemedicine
tools without much technical expertise. Caregivers should also have
access to the system to support patients and provide necessary
assistance.
Integration with medical professionals: The TM platform
should provide a well-structured and accessible interface for
physicians, nurses, and coordinators involved in the follow-up care
of ALD patients. It should allow for seamless communication, data
sharing, and collaboration within the health care team to ensure
coordinated and timely interventions.
Data analysis and predictive models: The TM system should
incorporate data analysis capabilities to process the collected
patient data and generate meaningful insights. Advanced analytics
and predictive modeling can help identify patterns, predict
complications, and provide personalized recommendations for
each patient.
Education and patient engagement: The TM approach should
include educational resources and interactive features to engage
patients in their care. This could involve providing educational
materials, personalized feedback, reminders for medications and
appointments, and opportunities for patients to ask questions or
seek clarification.
Scalability and accessibility: The TM system should be
designed to be scalable, allowing for easy expansion and adaptation
to accommodate a larger number of patients. It should also
consider accessibility factors such as language options, support
for individuals with disabilities, and compatibility with different
devices and Internet connectivity. Overall, the ideal TM approach
for patients with ALD should aim to provide continual monitoring,
early intervention, and personalized care in a convenient and
patient-centered manner, with effective communication and
collaboration among health care providers.
Useful Objective Measurements to Collect in Patients with ALD
Home spirometry
Forced Vital Capacity (FVC), considered a surrogate measure for mortality, is one of the most important outcome measures in ALD, and is used to guide treatment decisions in daily practice and clinical trials. Hypoxemia is another physiological feature of prognostic importance in ALD, and crucial for decision making around oxygen supplementation and titration. Six-minute walk tests and daily activity rates reflect a patient’s functional performance, while Patient-Reported Outcome Measures (PROMs) capture the direct effect of the disease on patient well-being and daily living. Coughing fits, one of the most common and disabling symptoms in ALD, can be measured with objective (e.g., recordings) and subjective (e.g., PROMs) methods. Home spirometry has gained increasing attention in the ALD field, with the first study published in 2016. A home spirometer is a handheld device that measures FEV1 and FVC. Several devices using different measurement technologies and methods to store and share data, and approved by the U.S. Food and Drug Administration are available. Ideally, home spirometers should be able to assess and provide feedback on test quality, measure inspiration and expiration, and do not require daily calibration. Modern devices are often connected to an online application (e.g., via Bluetooth) and use Wi-Fi for real-time data sharing with health care providers [25-27].
A few online applications with integrated home spirometry have been developed for pa-tients with ALD. Overall, patient satisfaction with online home spirometry is high. Though some applications have encountered technical problems (e.g., connectivity problems with the devices), online applications have the potential to improve adherence and reliability, with timely feedback from health care professionals. This feasibility and reliability was demonstrated in a Dutch home-monitoring program, which incorporated a technical helpdesk and an automated alert system for missing data or significant changes in measurements. In this study, adherence over time remained high, and slopes of home and hospital spirometry correlated well. With this approach, home spirometry can be valuable for daily care and clinical trials. There remains a need for standardized and validated technical requirements and analytical methods before home spirometry can be more widely implemented. Optimal frequency and timing for home spirometry measure-ments need further investigation, as testing frequency and diurnal variation could also affect results [2,28-34].
Pulse oximetry
Pulse oximeters have been one of the most widely used medical devices since their commercial introduction in the 1980’s, yet there was a scarcity of data on their use for home monitoring until the COVID-19 pandemic. Conventional portable pulse oximeters, which measure oxygen saturation on the basis of transmission and absorption of different light wavelengths, are inexpensive and simple to use. There are emerging wearable sensors (e.g., Biobeat Technologies, Petah Tikva, Israel) and mobile applications (e.g., DigiDoc Technologies, Egersund, Norway) that use photoplethysmography by measuring light re-flection for pulse oximetry monitoring. Newer devices allow continuous ambulatory oximetry monitoring in daily life, which can provide additional information of oxygenation status in patients with ALD to optimize oxygen supplementation, particularly when used with physical activity monitoring. However, available data regarding accuracy for several portable pulse oximeters and the newer systems are scant. Large reading errors have been shown in selected direct-toconsumer portable pulse oximeters that do not meet the standard set by International Organization for Standardization. In addition, other factors can affect pulse oximetry readings, including low perfusion, movement artifacts, darker skin pigmentation, and nail polish. Given that it is common to encounter patients with ALD who monitor oxygen saturation at home, there is a need to evaluate the use of, and support required for, reliable home use of pulse oximetry.
Activity trackers
Many different wearable devices are available for physical activity monitoring to objectively evaluate both the rates and habitual patterns of physical activity by use of step counts, activity intensity, and energy expenditure. These devices include pedometers, ac-accelerometers (e.g., ActiGraph, Pensacola, FL, USA) and gyroscopes, consumer-grade monitors (e.g., Fitbit, San Francisco, CA, U.S.A.), and smartphone applications for movement tracking, which use different signal transduction strategies for monitoring. Most devices record several physical activity variables besides steps per day, such as time standing, sedentary time, and sleep. The variables, or combinations of variables, that are of greatest clinical use in the assessment of patients with ALD remain unknown. Data from small studies in ALD show moderate associations between daily step counts and energy expenditure with lung function and 6-minute walk distance, and their prognostic potential for mortality. Daily step counts might be more sensitive than lung function in detecting disease progression in ALD [35-37].
Cough monitors
Objective evaluation with cough-frequency monitoring is currently used for research purposes in ALD, though its role in clinical practice has yet to be established. Nevertheless, objective monitoring with cough recorders is considered to be more reliable than patient recall and clinician appraisal in providing valid assessments of cough frequency, which correlate well with cough-related questionnaires. A 2021 study suggests that cough monitoring is feasible in the real-world setting and can provide useful clinical data, such as treatment response in patients with a chronic cough. The most widely used devices are the Leicester Cough Monitor (University Hospital Leicester, Leicester, UK) and the VitaloJAK (Vitalograph, Buckingham, UK). Both devices use a microphone for recording up to 24 hours to assess coughing during routine daily activities (Figure 1). The main drawback of these systems is the recording of environmental sounds, including private conversations. Though ambient sounds can be subsequently filtered out manually or with near-automated software, this could lead to privacy and confidentiality issues and a reluctance to wear the cough monitor. Furthermore, though these devices measure cough frequency, they do not generally provide any assessment of cough intensity [38-40]. Real-time monitoring and analysis of cough sounds with machine learning algorithms could overcome this obstacle. In 2019, a smartphone application was developed for continuous cough detection that showed similar performance to available cough recorders and manual counting, which can be combined with online administration of cough-related questionnaires.
Figure 1:Study design.

Patient-reported measures
Online collection with an application or other web-based platform can facilitate implementation of PROMs in daily care and in research. A task force of the professional society for health economics and outcomes research (formerly the International Society for Pharmacoeconomics and Outcomes Research) concluded that online PROMs have many advantages over paperbased completion of PROMs, and outcomes are valid and reliable. Compared with paper-based questionnaires, online administration of PROMs leads to better data quality, faster completion time, lower cost, and the ability to guide clinical decision making. Studies in patients with pulmonary fibrosis found that online administration of PROMs is feasible in this older patient population, and appreciated by patients for self-monitoring. Some studies advocate for the use of visual analogue scales or numeric rating scales instead of more lengthy PROMs [41]. Especially if completed online, these simple tools could provide added value for research and daily clinical practice by reducing missing values and allowing frequent data collection [42-46].
Hypotheses and Aims
Our research hypothesis for this proposal is that, though classic virtual teleconsulting is beneficial in the palliative care of patients with advanced lung disease, the costs and human resources involved are significant. Home telemonitoring based on few simple vital parameters, assessed automatically and continuously by an Artificial Intelligence (AI) system that is able to detect a worrisome trend and alert the clinical team whenever appropriate is effective and economically affordable.
Hence, the purposes of this research proposal will be:
a. To develop an AI-assisted telecare platform that enables
physicians to remotely monitor the situation of patients with
chronic diseases and support the data collection of lifestyle and
environmental factors from different sources.
b. To develop scalable modular respiratory chronic disease
prediction models for early prediction of acute exacerbations
using personal lifestyle factors, environmental factors, and
medical questionnaires to help patients improve disease
control.
c. To construct an appropriate Smartphone-Based Decision
Support System (DSS) to deliver personalized health evaluation
for patients and health professionals in order to achieve the
goal of precision health management.
In other terms, this study will analyze the effect of a telemonitoring pilot project on direct medical costs, health resource utilization, and mortality. Primary outcomes will be health care utilization, mortality, and medication use. Secondary outcomes will be health-related QoL, psychological morbidity, lung function, and cost-effectiveness.
Data on lifestyle, temperature, humidity, and fine particulate matter will be collected using wearable devices, a home air quality-sensing device, and a smartphone/tablet app. Acute exacerbation episodes will be evaluated post-hoc via standardized questionnaires. With these input features, we will evaluate the prediction performance of different machine learning models, including random forest, decision trees, k-nearest neighbor, adaptive boosting, and a deep neural network, combined with a nurse-based coordination desk that will call patients at home whenever the DSS alerts, and will involve the pulmonologist on call, if needed, to modify the therapeutic regimen in a timely way.
We will estimate incremental costs and effectiveness by comparing an ALD telemonitoring and an ALD standard care cohort over a period of 1 year. In doing so, we will address the limitations of existing studies in numerous ways. First, to the best of our knowledge, this will be the largest evaluation of ALD telemonitoring in Italy. A follow-up period of 1 year enables measuring mid-term outcomes reliably. Second, we will investigate the incremental causal effect of telemonitoring in pragmatic, routine clinical settings by using a combination of advanced data analysis techniques (e.g., machine learning) and a stepwise evaluation of the clinical issue at hand by isolating ALD-related from all-cause outcomes. As a result, we will be able to make precise judgements regarding the effectiveness of telemonitoring on respiratory-related outcomes. Finally, we will consider incremental costs in addition to effectiveness of the intervention, and will be the first to conduct an evaluation of telemonitoring for ALD in Italy.
Materials and Methods
Clinical setting: Study design
We will carry out a monocentric, assessor-blind, two-arm (intervention/control) randomized controlled trial. The allocation ratio between the intervention and control group will be 2:1. Bias will be minimized through randomization, allocation concealment, assessor blinding, and statistical adjustment or subgrouping during the analysis. The study is expected to have an overall duration of 18 months (first patient in, last patient out). The length of study per patient will be 12 weeks. The recruitment period will last several months.
Study population: Inclusion and exclusion criteria
During the study period, we anticipate enrollment of 20 patients diagnosed with ALD evaluated for lung transplantation. Primary respiratory issues will include COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria; in particular, people admitted to the hospital for an AECRF will be recruited when medically fit for discharge. Inclusion criteria will be as follows: ≥18 years of age, a confirmed clinical diagnosis of COPD from spirometry data in medical records (FEV1/FVC < 0.7), and an admission with a primary diagnosis of exacerbation of CRF.
Participants will be excluded if they have a visual or physical impairment preventing them from wearing the vest, e.g., wheelchair-bound or a psychological comorbidity preventing their participation, dementia, required palliative care or unable or unwilling to provide written informed consent. To ensure that our trial specifically tests the effect of the telemonitoring technology, intervention and control groups will be provided with the same clinical care (including self-management advice) according to the region in which they live. The only difference between the intervention and control groups will be the provision of the telemonitoring service (Figure 1).
Acute exacerbations definition
According to the World Health Organization (WHO) and the U.S. National Heart, Lung, and Blood Institute GOLD, an exacerbation is defined as “an event in the natural course of the disease characterized by a change in the patient’s baseline dyspnea, cough, or sputum that is beyond normal day-to-day variations, is acute in onset, and may warrant a change in regular medication in a patient with underlying COPD.” Though the definition is comprehensive, it raises a number of questions (e.g., what are “normal day-to-day variations”?), thereby limiting its adoption as a practical definition in the context of clinical studies. Therefore, the following definition of exacerbation, based on clinical experience and a comprehensive review of the literature, will be adopted: “An exacerbation is defined as a change in medication or an HCP contact (hospital admission or any documented contact, face-to-face contact or telephone calls) in the presence of a significant increase in symptoms.”
To measure respiratory failure symptoms, participants will be asked to complete the Exacerbation of Chronic Pulmonary Disease Tool (EXACT) each day. This 14-item patient-reported questionnaire evaluates breathlessness, cough and sputum, and chest symptoms [41,47], with the total EXACT score ranging from 0 to 100, and higher scores indicating greater symptom severity.
Service architecture
The service consists of an iOS/Android based smartphone app, wearable devices, an air quality sensing device, and modular prediction models. After patients are discharged from the hospital, all lifestyle and environmental key information will be effectively collected from a wearable device, an air quality sensing device, and a smartphone app. Then, real-time data will be displayed on the platform for medical staff to assist in decision-making. Modular prediction models will be immediately triggered on some very important abnormal vital signs, ensuring emergency safety and cost-effectiveness.
Medical smartphone app
The location-based personal health advice app will be developed for both Android and iOs platforms. This will allow the measurement of real-time streaming data such as heart rate, heart rate variability, acceleration, SpO2, respiration rate, steps, calorie consumption, and floors climbed via connection with wearable devices. A background location tracking feature and video chat will be activated after obtaining user data authorization. Since regular assessment and daily symptom records help physicians understand the patient’s disease condition, the app will provide a variety of chronic disease clinical questionnaires and symptom diary functions.
The interactive diary questionnaire allows patients to selfreport whether their symptoms have improved, become worse, or not changed compared with their understanding of what is “usual” for them. Chest tightness, breathlessness, sputum volume, and purulence will be treated as “major” symptoms, while having a cold or sore throat, and an assessment of feeling generally run down, will be treated as “minor” symptoms. Patients will also report whether they are taking relievers, antibiotics, steroids or any combination of the three. These data will be automatically uploaded to the server, after which physicians will provide personalized health promotion advice in real time through the data visualization platform.
Air quality sensing device
Patients with chronic respiratory diseases, such as chronic obstructive pulmonary disease and asthma, are particularly susceptible to air pollution. With the rapid progress of the Internet of Things, home environmental information can be detected by air quality sensors. In the present proposal, a CE-certified sensor for air quality will be used to collect fine particulate matter (PM2.5) levels, temperature, and humidity at home; these data will be uploaded automatically via a wireless network every hour.
Scalable AI-assisted telecare platform
Through the heretofore mentioned sources of information and communication technology methods, comprehensive patient data will be collected. To establish an effective connection between patients and physicians, the data platform will be designed to provide key information and trend charts to physicians and case managers, facilitating a rapid understanding of the patient’s current condition on one interface (through the use of a Dashboard). In addition to data visualization, this platform provides real-time warning functions to assist physicians and case managers in decision making. Physicians and case managers will set thresholds for abnormal vital sign warnings according to the patient’s status. When the vital signs exceed the thresholds, the platform actively triggers the health risk calculation process and notifies medical staff to intervene if necessary. Regarding the precision health management and prevention of chronic diseases, the platform will compute personal health risks based on modular chronic disease prediction models and the various collected data (Figure 2).
Figure 2:Graphical abstract.

Models for early prediction of acute exacerbation of chronic diseases
As mentioned, the baseline health risk value will be computed with a robust prediction model, and provided as decision support for physicians. The comprehensive dataset will be pre-processed to extract the key features, followed by the training process. The data pre-processing consists of the Last Observation Carried Forward (LOCF) interpolation for inconsistent frequency or null point, and re-sampling to deal with the disparate ratio of abnormal events. The normalized data will be analyzed with various models, and subjected to an external validation to ensure that models are reliable and applicable to different case groups in the real world.
Table 1:Useful tools and parameters to collect in patients with ALD.
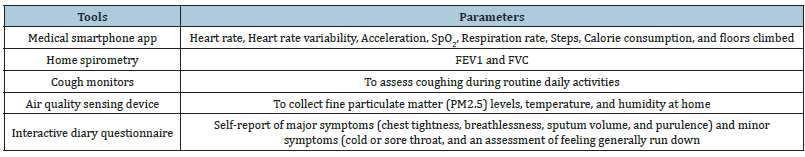
Hyperparameters for machine learning and the deep learning algorithm are presented in Table 1. Decision trees, random forests, linear discriminant analysis, and adaptive boosting will be used to implement the AECOPD prediction model. We also propose a deep neural network for comparison with machine learning methods. This will be constructed using fully connected layers, which connect each neuron in one layer to each neuron in another layer, mapping feature representations to the target vector space. For the activation function, we will use Rectified Linear Units (ReLU), with the introduction of a slope α, finishing with the sigmoid function to ensure a probability between 0 and 1. For the optimizer for updating parameters, Adaptive Momentum Estimation (ADAM) with quick parameter tuning and rapid convergence is suitable for many parameters. Though ADAM uses an adaptive learning rate, instead of using its decay function, for this model we will use an adaptive learning rate multiplied by 0.1 every 60 epochs. To account for the imbalanced data, we will use the class weights technique from Keras to penalize loss for categorizing data points as the wrong class.
Model assessment and validation
We will use 3-fold cross-validation to evaluate the stability of the prediction models. Accuracy, precision, sensitivity, and specificity will be used as assessment metrics to evaluate the overall performance, including the closeness and the deviation of the prediction, and the performance on negative and positive cases of the identification models based separately on the validation and test sets. To tune the models for the best performance on the test set, the F1 score will be computed to adjust and evaluate the performance of our multi-feature prediction tasks by varying the outcome thresholds using the validation dataset.
Feature engineering and model deployment
To deploy the prediction model in the real world, we will implement the SHapely Additive Explanations (SHAP) module, and a feature selection process to reduce the number of variables and the computational cost. The SHAP module is designed to explain the output of prediction models based on cooperative game theory, and determines the most important features and their influence on the model prediction. The formula of the SHAP value is defined in Equation 2. ϕi is the Shapley value for feature i. S is a coalition of features. p(S) is the payoff for this coalition. N is the total number of features. N/i is all the possible coalitions not containing i. In this study, a summary plot will be applied to describe the distribution and relationship of each feature. Furthermore, feature selection will be used to address overfitting and to find the best feature set for a useful, real-world prediction model. We will adopt the wrappers method and backward feature elimination to observe the performance change in precision, specificity, and F1 score. We will start the model with all features, and then remove insignificant features one by one until all features are processed. The resulting prediction model with the most cost-effective feature set will be deployed on our developed platform.
Conclusion
This project study aims to provide a comprehensive assessment of the telemonitoring pilot project’s impact on patient outcomes, health care resource utilization, and cost-effectiveness. Our research proposal is focused on comparing the effectiveness and cost-effectiveness of two approaches in care for patients with ALD: classic virtual teleconsulting and home telemonitoring with an AI system. The hypothesis suggests that while classic virtual teleconsulting may be beneficial, it comes with significant costs, and requires a substantial allocation of human resources. On the other hand, home telemonitoring, which relies on automatic assessment of vital parameters by an AI system, can effectively detect concerning trends, and alert the clinical team when necessary, all while being economically affordable. The results of this study may therefore be useful in understanding the real impact of a home monitoring system for patients with ADL.
Funding
This research was supported by EU funding within the Next Generation EU – MUR – National Recovery and Resilience Plan, Mission 4, Component 2 Investment 1.5 – Innovation ecosystem: Sicilian micro nanotech research and innovation center SAMOTHRACE (project no. ECS00000022, CUP B73D21014940004). This research was also supported by Italian Ministry of Health through Ricerca Corrente.
References
- Martin AB, Hartman M, Lassman D, Catlin A, National Health Expenditure Accounts (2021) National health care spending in 2019: Steady growth for the fourth consecutive year. Health Aff (Millwood) 40(1): 14-24.
- Hajat C, Stein E (2018) The global burden of multiple chronic conditions: A narrative review. Prev Med Rep 12: 284-93.
- Decramer M, Janssens W, Miravitlles M (2012) Chronic obstructive pulmonary disease. Lancet 379(9823): 1341-1351.
- Menn P, Heinrich J, Huber RM, Jorres RA, John J, et al. (2012) Direct medical costs of COPD-an excess cost approach based on two population-based studies. Respir Med 106(4): 540-548.
- Mathers CD, Loncar D (2006) Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3(11): e442.
- Achelrod D (2014) Policy expectations and reality of telemedicine - A critical analysis of health care outcomes, costs and acceptance for congestive heart failure. J Telemed Telecare 20(4): 192-200.
- Johannson KA, Lethebe BC, Assayag D, Fisher JH, Kolb M, et al. (2022) Travel Distance to subspecialty clinic and outcomes in patients with Fibrotic Interstitial lung disease. Ann Am Thorac Soc 19(1): 20-27.
- Pritzkuleit R, Beske F, Katalinic A (2010) Disease numbers in pneumology - A projection to 2060. Pneumologie 64(9):535-540.
- Salvati L, Palterer B, Parronchi P (2020) Spectrum of fibrotic lung diseases. N Engl J Med 383(25): 2485.
- Kruse C, Pesek B, Anderson M, Brennan K, Comfort H, et al. (2019) Telemonitoring to manage chronic obstructive pulmonary disease: Systematic literature review. JMIR Med Inform 7(1): e11496.
- Seewon Ryu (2012) Telemedicine: Opportunities and developments in Member States: Report on the second global survey on eHealth 2009. Healthc Inform Res 18(2): 153-155.
- Hekler E, Tiro JA, Hunter CM, Nebeker C (2020) Precision health: The role of the social and behavioral sciences in advancing the vision. Ann Behav Med 54(11): 805-826.
- Moller P (2020) The prospective lynch syndrome database reports enable evidence-based personal precision health care. Hered Cancer Clin Pract 18: 6.
- Gambhir SS, Ge TJ, Vermesh O, Spitler R (2018) Toward achieving precision health. Sci Transl Med 10(430): eaao3612.
- Ryan JC, Viana JN, Sellak H, Gondalia S, Callaghan NO, et al. (2021) Defining precision health: a scoping review protocol. BMJ Open 11(2): e044663.
- Goto T, Camargo CA, Faridi MK, Yun BJ, Hasegawa K, et al. (2018) Machine learning approaches for predicting disposition of asthma and COPD exacerbations in the ED. Am J Emerg Med 36(9): 1650-1654.
- Peng J, Chen C, Zhou M, Xie X, Zhou Y, et al. (2020) A machine-learning approach to forecast aggravation risk in patients with acute exacerbation of chronic obstructive pulmonary disease with clinical indicators. Sci Rep 10(1): 3118.
- Zeadally S, Bello O (2021) Harnessing the power of internet of things based connectivity to improve healthcare. Internet of Things-Neth 14: 100074.
- Howcroft M, Walters EH, Wood-Baker R, Walters JAE (2016) Action plans with brief patient education for exacerbations in chronic obstructive pulmonary disease. Cochrane Db Syst Rev 12(12): CD005074.
- Cruz J, Brooks D, Marques A (2014) Home telemonitoring effectiveness in COPD: A systematic review. Int J Clin Pract 68(3): 369-378.
- McLean S, Nurmatov U, Liu JL, Pagliari C, Car J, et al. (2011) Telehealthcare for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011(7): CD007718.
- Polisena J, Tran K, Cimon K, Hutton B, McGill S, et al. (2010) Home telehealth for chronic obstructive pulmonary disease: A systematic review and meta-analysis. J Telemed Telecare 16(3): 120-127.
- Pedone C, Lelli D (2015) Systematic review of telemonitoring in COPD: An update. Pneumonol Alergol Pol 83(6): 476-484.
- Henderson C, Knapp M, Fernandez JL, Beecham J, Hirani SP, et al. (2013) Cost effectiveness of telehealth for patients with long term conditions (Whole Systems demonstrator telehealth questionnaire study): Nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ 346: f1035.
- Johannson KA, Vittinghoff E, Morisset J, Lee JS, Balmes JR, et al. (2017) Home monitoring improves endpoint efficiency in idiopathic pulmonary fibrosis. Eur Respir J 50(1): 1602406.
- McCarthy K (2017) Selecting spirometers for home testing. Respiratory Therapy 12(4): 38-42.
- Russell AM, Adamali H, Molyneaux PL, Lukey PT, Marshall RP, et al. (2016) Daily home spirometry: An effective tool for detecting progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 194(8): 989-997.
- Khan F, Howard L, Hearson G, Edwards C, Barber C, et al. (2022) Clinical Utility of home versus hospital spirometry in fibrotic interstitial lung disease: Evaluation after INJUSTIS interim analysis. Ann Am Thorac Soc 19(3): 506-509.
- Moor CC, Gur-Demirel Y, Wijsenbeek MS (2019) Feasibility of a comprehensive home monitoring program for Sarcoidosis. J Pers Med 9(2): 23.
- Moor CC, Mostard RLM, Grutters JC, Bresser P, Aerts J, et al. (2020) Home monitoring in patients with idiopathic pulmonary fibrosis. A randomized controlled trial. Am J Respir Crit Care Med 202(3): 393-401.
- Moor CC, Berg CAL, Visser LS, Aerts J, Cottin V, et al. (2020) Diurnal variation in forced vital capacity in patients with fibrotic interstitial lung disease using home spirometry. ERJ Open Res 6(1): 00054-2020.
- Moor CC, Leuven SI, Wijsenbeek MS, Vonk MC (2021) Feasibility of online home spirometry in systemic sclerosis-associated interstitial lung disease: A pilot study. Rheumatology (Oxford) 60(5): 2467-2471.
- Moor CC, Wapenaar M, Miedema JR, Geelhoed JJM, Chandoesing PP, et al. (2018) A home monitoring program including real-time wireless home spirometry in idiopathic pulmonary fibrosis: A pilot study on experiences and barriers. Respir Res 19(1): 105.
- Noth I, Cottin V, Chaudhuri N, Corte TJ, Johannson KA, et al. (2021) Home spirometry in patients with idiopathic pulmonary fibrosis: data from the INMARK trial. Eur Respir J 58(1): 2001518.
- Bahmer T, Kirsten AM, Waschki B, Rabe KF, Magnussen H, et al. (2016) Clinical correlates of reduced physical activity in idiopathic pulmonary fibrosis. Respiration 91(6): 497-502.
- Bahmer T, Kirsten AM, Waschki B, Rabe KF, Magnussen H, et al. (2017) Prognosis and longitudinal changes of physical activity in idiopathic pulmonary fibrosis. BMC Pulm Med 17(1): 104.
- Drent M, Elfferich M, Breedveld E, Vries J, Strookappe B, et al. (2020) Benefit of wearing an activity tracker in sarcoidosis. J Pers Med 10(3): 97.
- Birring SS, Fleming T, Matos S, Raj AA, Evans DH, et al. (2008) The leicester cough monitor: Preliminary validation of an automated cough detection system in chronic cough. Eur Respir J 31(5): 1013-1018.
- Kvapilova L, Boza V, Dubec P, Majernik M, Bogar J, et al. (2019) Continuous sound collection using smartphones and Machine learning to measure cough. Digit Biomark 3(3): 166-175.
- Vertigan AE, Kapela SL, Birring SS, Gibson PG (2021) Feasibility and clinical utility of ambulatory cough monitoring in an outpatient clinical setting: A real-world retrospective evaluation. ERJ Open Res 7(4): 00319-2021.
- Leidy NK, Wilcox TK, Jones PW, Roberts L, Powers JH, et al. (2011) Standardizing measurement of chronic obstructive pulmonary disease exacerbations. Reliability and validity of a patient-reported diary. Am J Respir Crit Care Med 183(3): 323-329.
- Edwards C, Costello E, Cassidy N, Vick B, Russell AM, et al. (2020) Use of the patientMpower app with home-based spirometry to monitor the symptoms and impact of fibrotic lung conditions: Longitudinal observational study. JMIR Mhealth Uhealth 8(11): e16158.
- Meirte J, Hellemans N, Anthonissen M, Denteneer L, Maertens K, et al. (2020) Benefits and disadvantages of electronic patient-reported outcome measures: Systematic review. JMIR Perioper Med 3(1): e15588.
- Moor CC, Mostard RLM, Grutters JC, Bresser P, Wijsenbeek MS, et al. (2022) The use of online visual analogue scales in idiopathic pulmonary fibrosis. Eur Respir J 59(1): 2101531.
- Moor CC, Manen MJG, Tak NC, Noort E, Wijsenbeek MS, et al. (2018) Development and feasibility of an eHealth tool for idiopathic pulmonary fibrosis Eur Respir J 51(3):1702508.
- Scallan C, Strand L, Hayes J, Kadura S, Collins B, et al. (2022) R-scale for pulmonary fibrosis: a simple, visual tool for the assessment of health-related quality of life. Eur Respir J 59(1): 2100917.
- Crook S, Busching G, Keusch S, Wieser S, Turk A, et al. (2018) The association between daily exacerbation symptoms and physical activity in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis13: 2199-2206.
© 2023 Paolo Capuano. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






