- Submissions

Full Text
Trends in Telemedicine & E-health
The Need to Improve the Notification of Infectious Disease Systems in the UK–A Comparison of Four OECD Countries
Dr. Bongkyu Shin1*, Dr. Nadia Inglis2 and Dr. Steven Montgomery-Laird3
1Doctor, Warwick Hospital, UK
1Public Health Consultant, Interim Director of Public Health-Walsall Council, UK
1Steven Montgomery-Laird, Consultant Medical Microbiologist, University Hospital Coventry and Warwickshire, UK
*Corresponding author:Bongkyu Shin, Doctor, Warwick Hospital, UK
Submission: June 23, 2023; Published: September 13, 2023

ISSN: 2689-2707 Volume 4 Issue 4
Abstract
Reporting notifiable diseases is one of the keystones of modern disease surveillance and public health
policy making. The key requirements to achieve such coming from regular, accurate, and complete
reporting data. This, however, does not happen to the high rate of reporting as it should, resulting in
incomplete data and thus reducing the effectivity of disease surveillance for the purpose of public health
in the UK/England. A literature review was performed for the purpose of
a) Analyzing if other countries similar to the UK/England also under-report notifiable diseases and
b) Analyse if there are any traits which may be benchmarked in the UK/England to improve on the
completeness of reporting of notifiable diseases.
The papers returned from our searches showed that;
a) Under-reporting of notifiable diseases also occur in other countries, not only the UK,
b) Completeness of disease notification differ per disease type,
c) Electronic reporting is a preferred method of notification for the purposes of improving notification
rates, and
d) A greater effort in many countries is required to more clearly see the severity of under-reporting that
is occurring.
Introduction
Notifiable diseases are diseases or pathogens of concern which must be reported to the relevant government authorities [1] (Appendix 1). Whilst the exact type of diseases or pathogens may vary depending on each country, in general the diseases listed all pose significant threat to public health to warrant surveillance. This reporting of notifiable disease takes part of many bio- surveillance that governments undertake to ensure the four main objectives of disease surveillance are adhered to, which are: analysis of trends of disease, identification of persons with said disease, implementation of control measures, and monitoring of the effectivity of control measures [1-3]. These four objectives allow for the quick planning, response and evaluation of the evolving situation where a disease may be spreading through the population. Since it is known that early identification and early response can lead to a significant amount of difference in long term economic and health outcomes, it stands to reason that the upkeep of a good quality dataset is paramount [4]. This has been keenly demonstrated by pandemics in the past; most notably the recent ongoing SARS-CoV-2 pandemic [5,6].
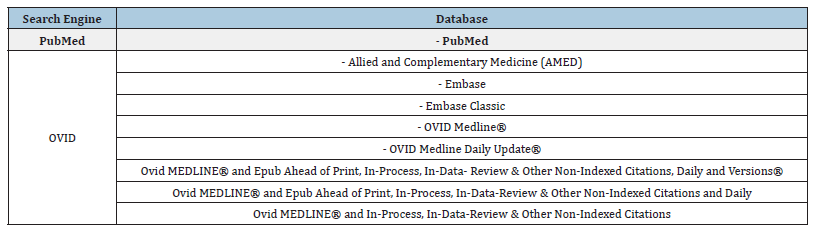
Appendix 1:PubMed and OVID databases.
In many countries, these reporting measures are achieved in 2
different routes.
a) Clinician reporting of suspected disease and
b) Laboratory reporting of listed organisms. Whilst
completeness of reporting tends to be higher in laboratory
reporting of listed organisms, the same is not true for clinician
reporting [7,8].
There is a wide range of completeness of reporting especially based on the disease type despite standing legal requirements [8]. Whilst there are many differing thoughts as to why this may be the case, the end result poses several problems in disease surveillance through the production of an incomplete dataset potentially undermining the severity of a rapidly evolving situation. It is with the above knowledge that we have undertaken this literature review to seek if other countries other than England suffer a similar problem.
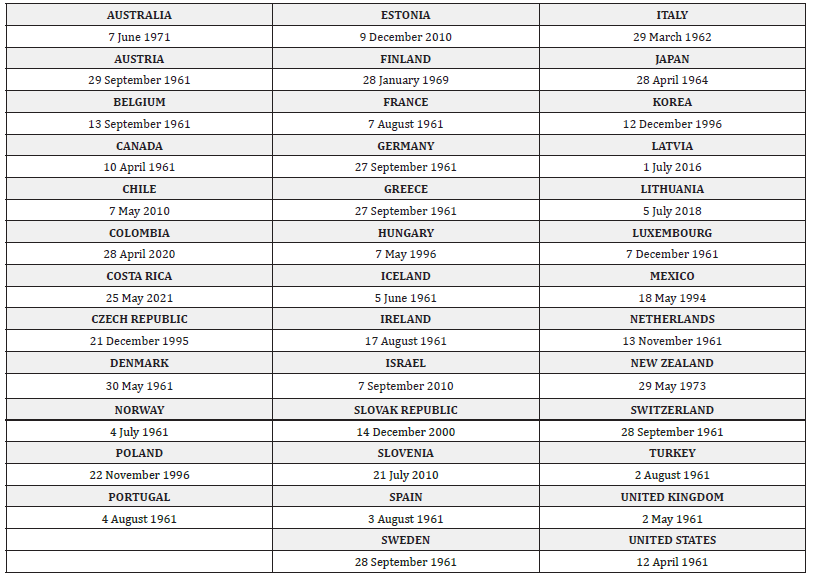
For the purposes of this review, we have limited our searches to three distinct countries within the listed OECD countries (Appendix 2) to allow for a comparison to be made with the current situation in England. The three countries identified were; Germany, Republic of Ireland and Republic of Korea. These three countries have been well established within the OECD for multiple years implying that they will have a similar goal towards economic development and better lives for their citizens [9] (Appendix 2). This allows for a good comparison of their respective completeness of reporting notifiable diseases as the major differences between said countries will be in health policies, specifically in disease notification. However, the three countries listed have distinctions in terms of regional, cultural and economic power/scale which will show if other factors alter completeness of notification. From this, we aim to see if the under-reporting of notifiable disease is prevalent in other similar countries to that of the UK/England and if not, find if there are aspects which improve reporting rate which may be benchmarked in the UK/England.
Appendix 2:OECD countries listed on the OECD website and their Respective dates of entry [43].
Method
Search strategy
A search was undertaken in PubMed and OVID under their databases (Appendix 1) with the aim of searching for papers relating to notifiable disease notification in the last two decades. This resulted in the following search criteria (Figure 1a & 1b).
Figure 1a:PubMed search criteria.

Figure 1b:OVID search criteria.

Exclusion criteria
From the onset, several exclusion criteria could be thought of considering the scope of the project. Any papers that pertained to countries outside of the OECD as of the search date (Nov 2021) (Appendix 2) were removed. Furthermore, any papers that were not reported in English was removed as well. Any papers pertaining to countries within the OECD were kept even if it was not part of the three main countries we were targeting. These were kept for reference purposes but were not counted for the purposes of analysis. Furthermore, all papers that pertained to ‘non-human’ subjects i.e. papers whose main focus was veterinary were removed as well as research papers (randomised control trials, case studies, novel qualitative or quantitative laboratory data reporting, governmental papers).
Filtering/screening
The papers which were obtained from the OVID and PubMed databases were extracted using the online tools available onto a csv file and then manually screened for; initially based on title then subsequently abstract and main body information. Papers were removed based on the lack of mentioning of any of the key words in the title: notifiable disease, disease notification, disease reporting, evaluation of, and notification system. Surviving papers were then screened for relevant information pertaining to actual notification rate of diseases and data on completeness of notification.
Result
Papers identified
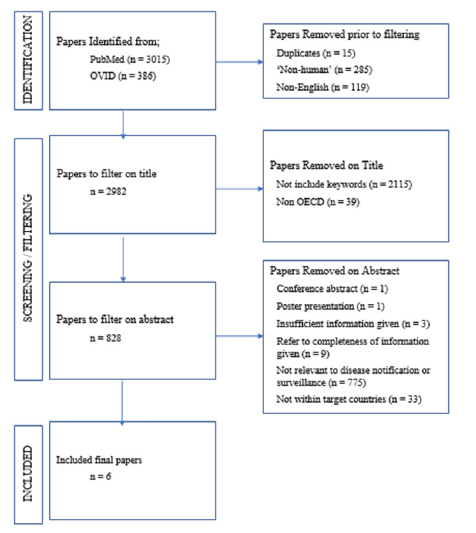
On initial search, a total of 3,401 results were returned from the OVID and PubMed databases. From these, 419 papers were removed for various reasons, the most common being that the main subject of the results returned were ‘non-human’ (n=285) i.e., concerning reporting of notifiable diseases for animal infections. A further 119 results were removed from the onset for not being in English and 15 results were removed for being duplicates either within or between databases.
Filtering on title
Of the surviving 2,982 results, a further 2,115 results were filtered out based on their title as per the exclusion criteria mentioned in the methods section as well as 39 results which were not part of the OECD. The vast majority of the results which were removed from the n=2,115 group was that of papers reporting novel experiments and datasets generated in laboratories.
Filtering on abstract
It should be noted that a number of results (n=9) referred to ‘completeness of reporting’ as that of completeness of the dataset that was reported e.g., patient identifiable detail (Date of Birth, Address, Demographics) or disease code/diagnosis [10-18]. A further 775 results failed to mention completeness of reporting notifiable disease within their abstract or main body or turned out to be papers reporting novel experiments and datasets. There were also instances of conference abstracts or poster presentations showing up in the databases (n=1 respectively). It was also at this stage that all OECD countries which were not part of the target 3+1 (Germany, Republic of Ireland, Republic of Korea, England) were removed (n=33).
Analysis of surviving results
In total, 6 papers were deemed acceptable and meeting all conditions as laid out in the methods [19-24] (Table 1). In all, each paper reports for different pathologies and the methods of analyzing the rate of reporting/completeness of reporting in different methods, with capture recapture being the most common (n=3) making any significant quantifiable data analysis difficult. However, on a broad view, it becomes apparent that from this dataset, which was generated through searches, under-reporting of diseases is also prevalent in other countries in the OECD.
Analysis on papers reporting on Tuberculosis (TB)
There were three papers reporting on the completeness of reporting of TB [19,21,23]. Of these three, two of the papers were from the UK/England and one from Germany. For these papers, one was a literature review, one utilised Capture Recapture (CR) to identify completeness of reporting [19] and one utilised record linkage [23]. The CR method utilised in the 2020 Domaszewska paper saw two data sources acquired; one containing ICD-10 diagnosis for TB, the other containing Prescriptions of Pyrazinamide (PZA) which is normally reserved for treating TB as a first line medication.
On seeing the rate of reporting for TB in these papers, it becomes apparent that in terms of TB, the UK/England underreports TB far more than Germany. Judging by how the UK papers with different methodologies have similar underreporting ranges for TB, we cannot assume that different methodologies are to fault for the reporting discrepancies.
Analysis on papers reporting on Infectious Meningococcal Disease (IMD)
There were two papers reporting on the completeness of reporting IMD [20,24]. These two papers reported on the completeness of reporting in England and the Republic of Ireland respectively stating that the rate of underreporting was -15.9% and -12.8% (Table 1). The England paper utilised capture-recapture analysis on two distinct datasets (Thames Valley Health Protection Unit, Health Protection Agency Meningococcal Reference Unit). The Ireland paper however, utilised record-linkage between Ireland’s Computerised Infectious Disease Reporting (CIDR) system and the Irish Meningitis & Sepsis Reference Laboratory (IMSRL) records to find their respective results. It becomes apparent that in the case of IMD reporting as well, UK/England shows indications of underreporting more than other countries in the OECD.
Table 1:Included final papers for review.
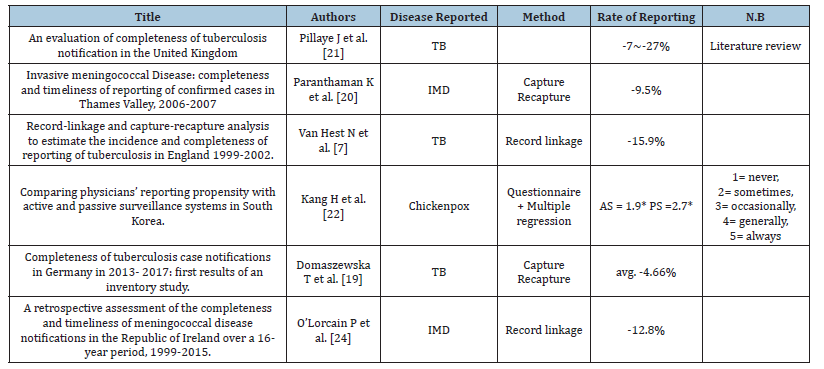
*Paper reported on the qualitative results of a questionnaire sent to physicians who were given a choice of describing how often they would notify chickenpox as per governmental regulations. 1=never reporting, 5=always reporting.
Analysis on papers reporting on Varicella Zoster (Chickenpox)
There was only one paper reporting on the completeness of reporting chickenpox [22]. Whilst the other papers were a quantitative study, this paper was achieved in the form of a questionnaire utilising a Likert scale. The scale ranging from 1-5 (1=never reporting, 5=reporting always) was used for both active and passive surveillance systems. Here, the authors referred to the Passive Surveillance system (PS) as the system that tends to be in place in many countries already, where clinicians and laboratories are mandated to report notifiable diseases. In an Active Surveillance system (AS), selective groups of clinicians and laboratories report for targeted diseases/pathologies. AS is also referred to as sentinels systems [22]. Here, the important aspect is not whether the AS is an improvement to the status quo of reporting and the completeness of reporting, but rather that in both AS and PS, physicians who have responded to the questionnaire (AS; n=62, PS; n=231) have responded as either sometimes reporting (AS) or occasionally reporting (PS) (Figure 2).
Figure 2:PRISMA flow diagram of the screening process.
Discussion
Comparing Differences in Reporting to Cultural/Systematic Differences between Countries as mentioned in the method section overall, there are two distinct differences between the completeness of reporting for the countries searched. One is the general underreporting of notifiable diseases in the UK/England, the second is the under-reporting of notifiable diseases in different countries which have varying levels of distinctness of cultures as set forth by Hofstede [25] (Appendix 3). From here, we can posit that there may be cultural and systematic differences between these countries which cause differences in the rate of reporting, despite all three under-reporting diseases.
Appendix 3:Cultural differences as applied by Hofstede’s cultural dimension theory; RoI=Republic of Ireland, RoK = Republic of Korea [25].

The reporting system within the UK/England is that of clinician or laboratory reporting of notifiable diseases to the proper local health authority or the UK Health Security Agency (UKHSA) (Previously known as Public Health England) respectively. This is a common system of reporting which is seen in other parts of the world such as that of Australia and the US as well as the three countries in question; Germany, Ireland, and Korea [26-30]. There are differences however, in the method of notification between the three countries and the UK/England. Within the UK/England and the Republic of Ireland, clinician reporting is still achieved by paper form or by telephone call if urgent to the local health protection team [31,32].
In other countries such as that of Germany and the Republic of Korea, an electronic reporting system is utilised for the purposes of both assessment and notification of diseases [29,33]. Unfortunately, despite the clear differences in procedure of reporting notifiable diseases in these four countries, due to the qualitative nature of the Korean study, and the lack of overall papers returned, we cannot state confidently from these datasets that one particular system leads to a better completeness of reporting. However, there are other studies which have already shown that electronic reporting systems result in an overall timelier and more complete reporting of notifiable disease [34-36].
In that regard, it is difficult to state that any cultural differences are the cause of different completeness of notification. To take an extreme example, whilst the Republic of Korea is indeed a far-removed nation to leave no uncertainty as to its cultural distinctness, considering how physicians conduct themselves to patient orientated care and are governed under procedural guidelines much like the UK/England, it is this authors opinion that cultural distinctness/differences do not hold as much weight as to the differences in the completeness of notification between different countries and cultures.
Problems with estimating under-reporting utilising Capture-Recapture (CR)
Capture-recapture is a common method utilised in ecology to
estimate population sizes of hard to count species [37]. However,
this methodology has also been applied in medicine to disease
incidence [38]. In order for CR to be utilised in estimating disease
incidence, two data sources are required and from there two
assumptions need to be made.
1. The data sources are independent and
2. The individuals have the same probability of being
captured by either/both sources. As Tilling states, these
assumptions are difficult to make in epidemiology as the ‘more
severe cases will be likely to be admitted to hospital and correctly
identified’.
This may explain what may be happening to some of the difference in the completeness of notifying diseases as listed on the UK government website where there is a difference in reporting for food poisoning and cholera, in comparison to some of the other disease listed e.g. rabies, tuberculosis, Sever Acute Respiratory Syndrome (SARS) as people are generally less likely to report to the hospital for the former [39]. That said, in the absence of a protocol to measure completeness to a similar standard without resorting to cross-referencing notification forms to individual patient records, CR remains to be in our opinion to be most efficient method of estimating population size of reported/non-reported individuals.
Difference in search criteria between PubMed and OVID
The search criteria specified a truncation for the terms ‘repo- ’ and ‘compl-’ so that any suffices that would follow would also have been included in the search results provided by PubMed and OVID. However, the truncation method for the two databases are different, with OVID utilising ‘$’ and PubMed utilising‘*’. This difference, however, should not alter any searches or cause significant deviations in the search parameters..
Number of Results Returned
As discussed in the results section, a total of six papers were returned from both databases. Given the self-imposed limitations of searching for OECD countries and specifically the three countries in question, the return of 39 papers and 6 papers for OECD countries and the three countries + UK/England seems to be an acceptable number. There are other databases such as the Cochrane library available but considering how the vast majority of medical papers are based on PubMed and Embase, we are fairly confident that the vast majority of papers have been searched for. That said, any significant comparison or analysis to the completeness of reporting cannot be reached from the number of papers returned. We are also confident that other countries in the OECD also suffer from underreporting of notifiable diseases [40,41] and also from countries outside the OECD [42,43].
To that extent, we can posit the following; a) under-reporting of notifiable diseases occurs not just within the UK/England but other countries in general, b) systematic differences, especially in electronic assessment and notification have a greater weight in under-reporting differences, c) more research is required in individual countries as to ascertain the degree of under-reporting that is occurring in order to draw any meaningful quantitative comparison between countries and pose a method of improvement.
Bias
Due to the nature of how the study was conducted, it cannot be denied that a form of selection and reporting bias could have been introduced. Whilst the master list of results generated by both databases were checked through by both the primary researcher and his clinical academic supervisor, the actual selection of the papers was undertaken solely by the primary researcher alone potentially generating reporting bias through incomplete identification of all data. Furthermore, due to the low number of papers that were eligible in the end, any conclusions that were drawn from the resulting papers were not made by the process of statistical analysis but rather postulations than concrete data.
Despite this, we feel that the potential biases have been well managed by the act of; a) running the search multiple times throughout the research period, and b) the final list of eligible papers being checked by the clinical academic supervisor (consultant microbiologist). It should also be noted that whilst the primary researcher is indeed a Korean national, the three countries to compare the UK/England to were chosen due to highest results number returned during a preliminary search as well as their diversity in their systematic and cultural sides.
Conclusion
In conclusion, under-reporting of notifiable diseases is a wellknown occurrence that occurs not only in countries similar in policies and cultures to the UK, but also culturally distinct countries with more advanced methods of reporting as well. However, to quantify the amount of under-reporting that occurs in different systems, a greater effort is required to analyse the amount by which under-reporting occurs.
Only then would we be able to see if there exists a difference
of a statistically significant amount and from there would we be
able to analyse and benchmark improvements to be made in the
UK. Despite this, there are some clear conclusions that can be made
at this stage.
a) electronic reporting is a method which is necessary
and will improve rates of reporting regardless of cultural
differences,
b) under-reporting differences per disease type and
electronic assessment and reporting will improve on underreporting
of specific diseases.
References
- Wagner MM, Moore AW, Aryel RM (2005) Handbook of biosurveillance. 1st (edn), Elsevier, Netherlands.
- Nsubuga P, White ME, Thacker SB, Anderson MA, Blount SB, et al. (2006) Public health surveillance: A tool for targeting and monitoring interventions. Jamison DT, Breman JG, et al. (Eds.), Dis Control Priorities Dev Ctries. 2nd (edn), Oxford University Press, Washington, (DC): The International Bank for Reconstruction and Development/The World Bank, New York, USA.
- (2012) Principles of epidemiology in public health practice. 3rd (edn), An introduction to applied epidemiology and biostatistics. Lesson 5, Section 2, Self-Study Course SS1978, CDC.
- Madhav N, Oppenheim B, Gallivan M, Mulembakani P, Rubin E, et al. (2017) Pandemics: risks, impacts, and mitigation. 3rd (edn), Disease Control Priorities: Improving Health and Reducing Poverty. The International Bank for Reconstruction and Development/The World Bank, California, USA.
- Budd J, Miller BS, Manning EM, Lampos V, Zhuang M, et al. (2020) Digital technologies in the public-health response to COVID-19. Nat Med 26(8): 1183-1192.
- Ienca M, Vayena (2020) On the responsible use of digital data to tackle the COVID-19 pandemic. Nat Med 26(4): 463-464.
- Vanhest NAH, Story A, Grant AD, Antoine D, Crofts JP, et al. (2008) Record-linkage and capture-recapture analysis to estimate the incidence and completeness of reporting of tuberculosis in England 1999-2002. Epidemiol Infect 136(12): 1606-1616.
- Dixon BE, Zhang Z, Lai PTS, Kirbiyik U, Williams J, et al. (2017) Completeness and timeliness of notifiable disease reporting: a comparison of laboratory and provider reports submitted to a large county health department. BMC Med Inform Decis Mak 17(1): 87.
- (2022) About the OECD-OECD.
- Vogt RL, Spittle R, Cronquist A, Patnaik JL (2006) Evaluation of the timeliness and completeness of a web-based notifiable disease reporting system by a local health department. J Public Health Manag Pract 12(6): 540-544.
- Garcia MC, Garrett NY, Singletary V, Brown S, Hennessy BT, et al. (2018) An assessment of information exchange practices, challenges, and opportunities to support US disease surveillance in 3 States. J Public Health Manag 24(6): 546-553.
- Brandwagt DAH, Ende AVD, Ruijs WLM, Melker HE, Knol MJ (2019) Evaluation of the surveillance system for invasive meningococcal disease (IMD) in the Netherlands, 2004-2016. BMC Infect Dis 19(1): 1-8.
- Nicolay N, Garvey P, Delappe N, Cormican M, McKeown P (2010) Completeness and timeliness of Salmonella notifications in Ireland in 2008: A cross sectional study. BMC Public Health 10(1): 1-8.
- Miller M, Roche P, Spencer J, Deeble M (2004) Evaluation of Australia’s national notifiable disease surveillance system. Commun Dis Intell Q Rep 28(3): 311-323.
- Gibney KB, Cheng AC, Hall R, Leder K. Australia’s National notifiable diseases surveillance system 1991-2011: expanding, adapting and improving. Epidemiol Infect 145(5): 1006-1017.
- Ward M, Brandsema P, Straten EV, Bosman A (2005) Electronic reporting improves timeliness and completeness of infectious disease notification, the Netherlands. Euro Surveill 10(1): 7-8.
- Matsui T, Kramer MH, Mendlein JM, Osaka K, Ohyama T (2000) Evaluation of national tsutsugamushi disease surveillance--Japan, Jpn J Infect Dis 55(6): 197-203.
- Boes L, Houareau C, Altmann D, Heiden MD, Bremer V, et al. (2020) Evaluation of the German surveillance system for hepatitis B regarding timeliness, data quality, and simplicity, from 2005 to 2014. Public Health 180: 141-148.
- Domaszewska T, Karo B, Preuss U, Kollan C, Reuss A, et al. (2020) Completeness of tuberculosis case notifications in Germany in 2013-2017: first results of an inventory study. BMC Infect Dis 20(1): 1-13.
- Paranthaman K, Kent L, McCarthy N, Gray SJ (2009) Invasive meningococcal disease: completeness and timeliness of reporting of confirmed cases in Thames Valley, 2006-2007. Public Health 123(12): 805-808.
- Pillaye J, Clarke A. (2003) An evaluation of completeness of tuberculosis notification in the United Kingdom. BMC Public Health 3(1): 1-5.
- Kang HY, Shin E, Kim YS, Kim JK (2014) Comparing physicians’ reporting propensity with active and passive surveillance systems in South Korea. J Korean Med Assoc 57(2): 167-175.
- Van NAH, Story A, Grant AD, Antoine D, Crofts JP, et al. (2008) Record-linkage and capture-recapture analysis to estimate the incidence and completeness of reporting of tuberculosis in England 1999-2002. Epidemiol Infect 136(12): 1606-1616.
- O’Lorcain P, Bennett DE, Morgan SL, Cunney RJ, Cotter SM, et al. (2018) A retrospective assessment of the completeness and timeliness of meningococcal disease notifications in the Republic of Ireland over a 16-year period, 1999-2015. Public Health 156: 44-51.
- Compare countries-Hofstede Insights.
- What is case surveillance? Notifiable Diseases and Conditions. CDC, National Notifiable Diseases Surveillance System (NNDSS).
- Krause G, Ropers G, Stark K (2005) Notifiable disease surveillance and practicing physicians. Emerg Infect Dis 11(3): 442-445.
- National Notifiable Diseases surveillance System (NNDSS), National notifiable diseases surveillance infographic, CDC.
- Park S, Cho E (2014) National infectious diseases surveillance data of South Korea. Epidemiol Health. 36: e2014030.
- Notifiable diseases. Health Protection Surveillance Centre, Ireland.
- Notifiable diseases: form for registered medical practitioners-GOV. UK.
- Who to notify. Health Protection Surveillance Centre, Ireland.
- Zucs AP, Benzler J, Krause G (2005) Mandatory disease reporting by German laboratories: a survey of attitudes, practices and needs. Euro Surveill 10(1): 26-27.
- Ward M, Brandsema P, Straten EV, Bosman A (2005) Electronic reporting improves timeliness and completeness of infectious disease notification, The Netherlands, 2003. Euro Surveill 10(1): 27-30.
- Progress in improving state and local disease surveillance---United States, 2000-2005. MMWR Morb Mortal Wkly Rep 54(33): 822-825.
- Automated detection and reporting of notifiable diseases using electronic medical records versus passive surveillance --- massachusetts, June 2006--July 2007. MMWR Morb Mortal Wkly Rep 57(14): 373-376.
- Corliss DJ (2019) Capture-Recapture Databases for Data For Good Projects, pp. 1-10.
- Tilling K (2001) Capture-recapture methods-useful or misleading? Int J Epidemiol 30(1): 12-14.
- (2010) Notifiable diseases and causative organisms: how to report-GOV.UK.
- Bennett SEE, Weber DJ, Poole C, MacDonald PDM, Maillard JM (2011) Completeness of communicable disease reporting, North Carolina, USA, 1995-1997 and 2000-2006. Emerging Infectious Diseases journal 17(1): 23-29.
- Wolff C, Lange H, Feruglio S, Vold L, MacDonald E (2019) Evaluation of the national surveillance of Legionnaires’ disease in Norway, 2008-2017. BMC Public Health 19(1): 1624.
- Benson FG, Levin J, Rispel LC (2018) Health care providers’ compliance with the notifiable diseases surveillance system in South Africa. PLoS One 13(4): e0195194.
- List of OECD member countries-ratification of the convention on the OECD.
© 2023 Dr. Bongkyu Shin. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






