- Submissions

Full Text
Trends in Telemedicine & E-health
Meal Replacements in Conjunction with an Integrated, Technology-Driven Strategy for Treatment of Patients with Diabetes in Rural, Underserved Areas: A Pilot Study
Richard J Santen1*, Cindy Cunningham2, Carla Horton3, Ralf Nass1 and Wei Yue1
1Department of Medicine, Division of Endocrinology and Metabolism, USA
2Martinsville Henry County Coalition for Health and Wellness, USA
3Tri-Area Community Health Centers, USA
*Corresponding author:Richard J Santen MD, Division of Endocrinology and Metabolism, University of Virginia Health System, Charlottesville, USA
Submission: March 09, 2023; Published: March 21, 2023

ISSN: 2689-2707 Volume 4 Issue 1
Abstract
Background: Rural, medically-underserved, financially-challenged geographical areas lack
endocrinologists to provide consultation and management of patients with uncontrolled diabetes.
Our prior studies in 139 patients with diabetes attending rural, Federally Qualified Community Health
Centers, demonstrated the utility of telemedicine to link patients with endocrinologists and markedly
improve glycemic control. However, a critical limitation was the inability to achieve weight reductions,
even with nutritional education.
Study Design: This pilot study utilized meal replacements linked to an integrated, technology-driven
strategy for management of patients with uncontrolled diabetes in underserved areas. Primary and
secondary goals: Our primary goal was weight reduction and secondarily, improved glucose control.
Result: Eleven patients completed 3 months of the program and 7, the entire 6 months. In the 11 patients
evaluated at three months, body weights dropped from 271±16 to 252±11 (SEM) pounds (P=0.002). The
meal replacement diet was associated with a decrease in insulin requirements with doses falling from
221±41 to 129±23 (SEM) units daily (P=0.004). Hemoglobin A1c levels declined from 9.85±0.33% to
7.93±0.31% (SEM) (p=0.001). In the 7 patients completing the entire 6 months of the protocol, similar
reductions persisted. Mean compliance with meal replacements on a scale of 0 to 10 ranged downward
from 8.6 to 7.6 (P=NS) with increasing duration of study.
Conclusion: The study was unique in tailoring a program specifically to rural, underserved areas and
demonstrated weight, insulin dose, and HbA1c reductions. The data now require confirmation of the use
of meal replacements linked to multiple technologies in a future, randomized trial.
Keywords:Nutrisystem®D® meal replacement; Glucommander-outpatient; Telcare meter; telemedicine; Remote consultation; Federally qualified community health centers
Abbreviations: CDCES: Certified Diabetes Care and Education Specialists
Introduction
Patients with diabetes mellitus living in rural, economically challenged, medically underserved areas generally lack endocrinologists for consultative evaluation and management [1]. This deficiency of specialists has resulted primarily from a marked workforce gap of endocrinologists in the United States which is particularly evident in underserved, rural areas [2,3]. Multiple social, economic, educational, geographical, and transportation challenges are involved in the treatment of patients in these rural areas as extensively reviewed previously [1,4-11]. Telehealth has been used as a means to improve the care of patients with diabetes in general as well those living in rural areas. [6,7,10,12-29].
Over the past five years, our team has attempted to overcome this gap in five rural clinics by means of telehealth, frequent phone encounters, and educational sessions coordinated by two academic endocrinologists [30]. Our comprehensive team included endocrinologists, Certified Diabetes Care and Education Specialist (CDCES), nutritionists, nurse practitioners, physicians and administrative staff at the participating clinics. Our program provided comprehensive management of patients with diabetes attending Federally Qualified Community Health Centers and was successful in reducing HbA1C levels. Weekly phone calls with insulin instructions were considered important for gaining patient compliance. Importantly, however, mean body weights did not decrease [30]. As a recent emphasis in diabetes care has been on weight reduction as a primary goal, we recognized the need to design and implement additional measures to achieve this endpoint [31-33].
Based on our prior experience with telemedicine and technology, we designed a pilot program to enhance the ability of patients to lose weight and achieve improved glucose control. We postulated that utilization of meal replacements with restricted calories would facilitate weight loss and provide an excellent method to educate patients about caloric content and portion control [34-38]. We also utilized specific technologic approaches enabling us to work around several problems unique to rural areas encountered in our prior study. Specifically, as internet access is limited in the rural areas served, we devised a method of overcoming this problem by use of Telcare glucose meters [39] which store data and upload it when the patients drive their vehicles within the range of cell towers. The Telcare program also utilizes electronically enabled blood pressure cuffs and scales which allow transfer of data to the cloud [30].
The study design took into account the fact that the time
commitment needed to make weekly decisions about insulin
adjustments could be limiting. We hypothesized that the
glucommander- outpatient system would allow use of algorithms
to determine insulin doses and ultimately could be employed by
non-specialized providers, nurses or pharmacists [40,41]. For this
reason, we utilized this system to make effective recommendations
about insulin dosing. Employing each of these components, we
wrote up a detailed research protocol and obtained approval
for it by our institutional review board. Six key elements of the
technology included:
A. Nutrisystem®D® meal replacements as a means to
educate about calorie intake and meal composition
B. The glucommander-outpatient algorithmic system to
determine insulin dosages
C. Telcare meters, weight scales, and BP cuffs to store
glucose, weight and blood pressure data and upload to the
cloud
D. DEXCOM continuous glucose monitoring to ensure
safety and lack of hypoglycemia at the start of use of meal
replacements
E. iPads containing four 2 hour educational sessions
F. A cloud based movie describing the program to provide
the specific details of each step to patients.
We report here that this series of applicable technologies proved beneficial in the management of patients. Body weight, HbA1c levels and insulin doses fell statistically significantly at 3 and 6 months compared to baseline and without substantial incidence of hypoglycemia. The data suggested the potential utility of this technology-driven program and provided sufficient data to support initiating a large randomized trial in the future. The goal would be to determine whether the approach used would be superior to usual care in treating patient with diabetes in rural underserved areas.
Methods
Clinics: Federally Qualified Community Health Centers provided the source of patients, financial support for critical personnel involved in the program, and for infrastructure (Figure 1). Two separate organizational structures were utilized (1) The Tri-Area Community Health Center Program involved clinics at Laurel Fork, Ferrum and Floyd, Virginia and (2) The Henry County Coalition for Health and Wellness included clinics at Bassett and Ridgeway, Virginia. As reported previously, these clinics are located in rural, underserved areas where the median family income is well below the average for the Commonwealth of Virginia [30].
Figure 1:Characteristics of federally qualified community health centers.

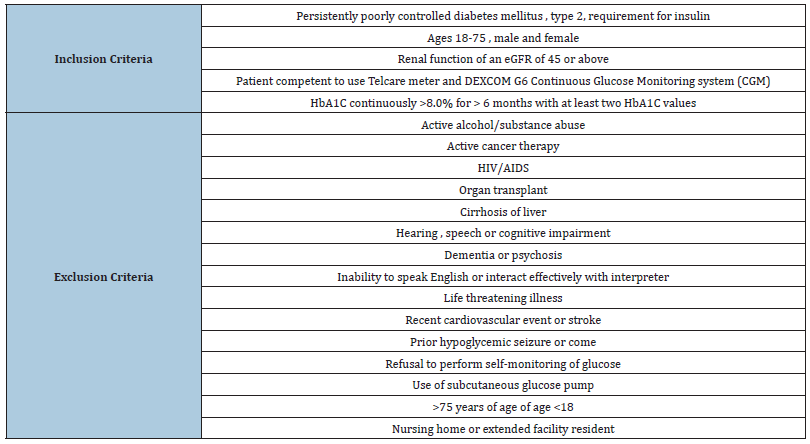
Patients: Individuals with type 2 diabetes who met the inclusion and exclusion criteria listed in Table 1 were asked to volunteer for the study. These included 7 women and four men with a mean age of 59 (range 42 to 73).
Table 1: Inclusion and exclusion criteria for the study..
Evaluations: Prior to the initial one hour telemedicine evaluation, comprehensive records were sent electronically to the two endocrinologist-co-authors (RJS and RN). The telemedicine evaluation visits took place prior to initiation of the study and utilized a standard form to obtain a comprehensive history. Vital signs, demographic and lifestyle information were obtained by coauthors (CC and CH) who were CDCESs (Certified Diabetes Care and Education Specialists). The physical exam data were taken from the records of exams performed by the primary care providers. Laboratory data from prior clinic visits were at that time reviewed. A detailed consult report was then dictated, reviewed, and sent to the primary care provider. Data in the report included history, physical exam, assessment, recommendations and the specific laboratory tests requested.
Laboratory data:Prior to study entry and at three and six months, blood was obtained for HbA1C, comprehensive metabolic panel, lipid profile, TSH, and CBC. Initial education for technology use: The patients were taught by the co-authors, CC and CH, in the clinic to use the Telcare meter, blood pressure cuff and electronic scales as well as the process that the system used to transfer that data to the cloud.
Follow-up diabetes and nutrition educational sessions:Four, two hour educational sessions were provided to the patients
which were uploaded on to Apple iPADs as developed by the UVA
nutrition team and supervised by a nutritionist.
The four topics included:
A. The basics of diabetes.
B. Nutrition basics.
C. Diabetes self-management skills.
D. Healthy eating lifestyle changes.
Telcare process:This data collection system uploaded finger stick glucose measurements, blood pressure results, and body weights into the Telcare meter. When the patient had either immediate access to the internet or drove their vehicle within range of a cell tower while carrying the meter, the data were uploaded to the cloud. The Telcare data were available to the investigators by accessing the cloud based web site. In order for access to the algorithm for insulin dosage decisions, glucose data were transferred electronically to the Glytec based glucommander– outpatient website. Blood pressures and body weights were not transferred but were accessed on the Telcare web site.
Glucommander-outpatient process:The Glytec web site displayed the recent average and time dependent glucose levels and suggested insulin doses. The system allowed overrides and mechanisms to alter suggested doses. The investigators accessed these data weekly and called patients on the telephone to make recommendations (Figure 2).
Figure 2:Example of the data provided in the glucommander-outpatient dashboard.

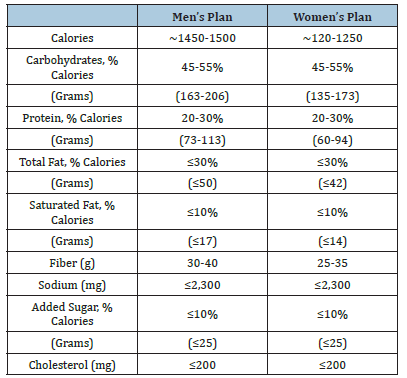
Nutrisystem®D® Meal Replacements:The patients selected the food that they wished to eat and communicated this to Nutrisystem Inc, for construction of the Nutrisystem®D® diets. These consisted of an average of 1450 to 1550 calories per day for men and 1200 to 1250 calories per day for women. The Nutrisystem®D® plan includes Nutrisystem®D® breakfasts, lunches, dinners and snacks-2 snacks for male plans and 1 snack for female plans. Using guidelines from Nutrisystem®D® instructions, patients supplement their Nutrisystem®D® food with fresh grocery food add-ins to complete the daily meal plan. The full composition of these diets for men and women is shown on Table 2.
Table 2: Composition of the meal replacement diets.
Safety measures:The patients utilized a DEXCOM Pro for 10 days at the start of the study and again at 3 and 6 months. These provided information about the percentage of very low and low glucose values during these times of the study.
Compliance data:At the weekly phone calls, the patients were asked to assess the degree of compliance subjectively, using a scale of 0 to 10 with 10 representing complete compliance.
Communications:The two CDCESs participating in the study (CC and CH) discussed the changes in insulin doses with the referring primary care providers who then ordered the insulin prescriptions as requested. The patients were called by telephone once weekly to review the glucomander-outpatient data and make the changes in insulin dosage recommended by the glucommanderoutpatient algorithm.
Protocol approval:The study was approved by the University of Virginia Institutional Review Board and written informed consent was obtained from all subjects. An IRB approved movie explaining all of the procedures was shown to each potential subject prior to obtaining informed consent.
Result
In planning this study, we anticipated that the marked reduction in calories engendered by the Nutrisystem®D® diet would cause an acute, potentially dangerous reduction in glucose levels. Accordingly, we utilized the DEXCOM Pro continuous glucose monitoring system during the first ten days of the study to identify hypoglycemic episodes. In the first patient, the hourly glucose levels monitored closely fell rapidly from 220 to 85mg% on the first day of the study. In response, the insulin dose was reduced by 50% from baseline values in this patient and then routinely in all subsequent participants at the start of the Nutrisystem®D® diet. With this adjustment, during the first ten days on the diet, episodes of very low glucose levels (i.e <54mg %) occurred in only two patients (0.7 and 1.6 percent of measurements) and low values (i.e 54-69mg %) in 5 patients (3.3, 1.4, 1.3, 3.7 and 4.8% of measurements). These hypoglycemic episodes were correctly quickly with oral glucose supplementation.
The goal of statistically significant weight loss was achieved. In the 11 patients evaluated at three months as body weights dropped 7.1% from 271±16 to 252±11 (SEM) pounds (P=0.002) and persisted in the 7 patients completing the study (245±20) (SEM) pounds. (P=0.009) (Figure 3). During the entire course of the study, insulin doses were given precisely according to the algorithm with no exceptions. At each week’s phone call, the glucommanderoutpatient logs were reviewed on line see example in Figure 2. The data illustrate the individual glucose values, the average blood glucose levels, the glucose levels before breakfast, lunch, dinner and bedtime and the suggested insulin doses to be administered as shown. The meal replacement diet and associated weight loss were reflected by a chronic drop in insulin requirements with doses falling 42% from 221±41 at baseline to 129±23 (SEM) units daily (P=0.004) at three months and persisting at 6 months (Figure 3).
Figure 3:Data from the baseline and at three and six months in the subjects entered into the study.
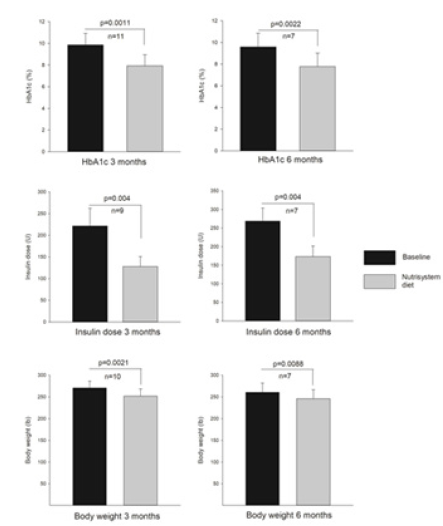
Following the weekly insulin recommendations, as shown in Figure 2, the baseline hemoglobin A1C levels fell significantly. In the 11 patients completing the three month protocol, the HbA1C decreased from 9.85±0.33% at baseline to 7.93±0.31% (SEM) (P=0.004). In those completing the entire six months, the levels dropped from 9.60±0.49% at baseline to 7.77±0.47% (SEM) (P=0.004). Hypoglycemia at very low levels occurred in no patients at three months as detected by Dexcom and low values occurred in only one patient in 0.1% of measurements. At six months, no patients had very low values and one had low values on 1.4% of the measurements.
Lipid levels did not changes significantly when comparing baseline with 6 months. Total cholesterol was 150±17 at baseline and 142±17 (SEM) at 6 months. Similar comparisons for HDL cholesterol were 38.8±4.0 versus 36±2.7 (SEM); for LDL-cholesterol 80.8±13 versus 75.6±14 (SEM); and for triglyceride 208±21 versus 18±29 (SEM) with all P values non-statistically significant. Blood pressure levels also did not change significantly averaging 154/89 at baseline and 152/86 at 6 months in the 7 patients completing the protocol.
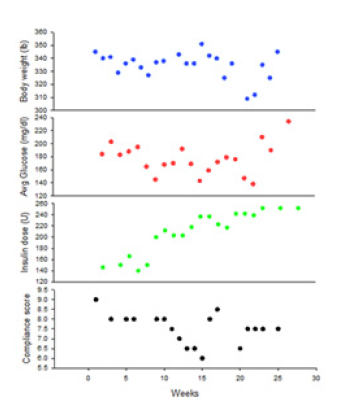
Compliance was highly variable and appeared likely to influence both body weight and insulin levels. Mean levels of compliance during weeks 1-4, 5-12, 13-20, and 21-24 were respectively 8.85, 8.61, 7.66, an 8.0. Figure 4 provides anecdotal data in a single patient to illustrate how variations in compliance can affect insulin levels and body weight.
Figure 4:Example in one patient of level of compliance, body weight, average glucose, an insulin dose over the duration of the protocol.
Discussion
This pilot study was designed to test whether a commercial meal replacement system, in conjunction with several technologic strategies, could result in weight loss. Notably, weight reduction was not accomplished in our previous five year study using telemedicine to manage patients with diabetes in rural, financially challenged, underserved areas [30]. Our prior published study, involving 139 patients, demonstrated substantial improvement in glycemic control but importantly, no statistically significant weight loss. Accordingly, we designed this pilot trial which introduced meal replacements and multiple telehealth technologies to facilitate the effective management of this population of patients by academically based endocrinologists. Our primary goal was to reduce body weight and secondarily, HbA1C levels. The combination of meal replacements and the additional 5 technological components resulted in meeting our goal of statistically significant reductions in weight loss, insulin dosages and HbA1C levels (Figure 3). Taken together, these methods allowed achievement of important biologic effects as documented by the marked reduction in insulin dosages and HbA1c levels.
While not proven, it is likely that the Nutrisystem®D® diets contributed substantially to the reduction in body weights observed (Figure 3). This contention is based on the comparison of the current results using meal replacements where weight reduction occurred and data in our prior study where weights remained stable. Additional support for this conclusion comes from two published studies with prior use of the Nutrisystem®D® program in patients with diabetes which resulted in similar weight losses as in this study [35,42]. Admittedly, a separate research trial would be required to more directly determine the specific effects of the Nutrisystem®D® diet independently of the technological components we utilized here. We recognize that it would not be practical to utilize these meal replacement diets on a longterm basis. Their utility, in our opinion, is to provide an ideal method of education about portion sizes, the role of snacks and the composition of diets. As our patients had uniformly obtained nutrition education sessions previously, we tentatively concluded that the Nutrisystem®D® approach is a more effective means of education.
It was expected that patients would be variably compliant in adhering to the Nutrisystem®D® diets. A subjective scoring system confirmed this supposition as the degree of compliance among patients and by the same patient (Figure 4) was variable. Patients commented that the food was good tasting but became somewhat repetitive over time. This could have reduced compliance as the duration of the study progressed. The data suggested but did not statistically confirm that the degree of compliance did diminish during the later months of the protocol. Based on this concept, we speculate that a 3-month use of the Nutrisystem®D® diet might be sufficient for patient education and the weight loss observed at that time period [35,42].
One key to the success of our prior and the current program appeared to be the interaction with patients on a weekly basis [43]. This facilitated rapid changes in insulin dosage with resultant improvement in glucose levels which appeared to motivate patients. While this pilot study utilized board certified endocrinologists, the use of the glucommander-outpatient system could easily be used by nurses or pharmacists as no changes were made in the insulin doses suggested by the glucommander-outpatient algorithm. For practical implementation of a program such as the one described, non-physician health care workers will likely be necessary to manage weekly phone calls [43]
Some of the benefits of the program occurred before the six month, planned termination date. For that and other reasons only 7 of the 11 patients entering the study completed it. One patient achieved excellent glycemic control within three months and did not see the benefit of continuing. Another decided to undergo bariatric surgery and a third could not adequately learn how to use the telcare meter and DEXCOM CGM. A fourth was lost to follow up for unknown reasons.
Our previously published program utilized a partially retired endocrinologist (RJS). From this experience, we hypothesized that retired endocrinologists could be enlisted to re-enter practice and help to improve the work force gap which exists regarding trained endocrinologist to manage patients with diabetes. Termed “rebooting the endocrinologist”, a program utilizing such individuals could also utilize a program such as that described here [30,44]. The template provided by this study could then be widely applied, particularly for Federally Qualified Health Centers in rural, underserved financially challenged areas.
In conclusion, while this pilot study involved relatively few patients, the reductions in weight, insulin dosage and HbA1c levels were highly statistically significant. We provided substantial evidence that meal replacements and use of multiple technological methods can be used to improve the care of patients with diabetes, living in rural underserved areas. This concept now requires confirmation in a large, randomized, controlled trial to determine if this approach is superior to usual care.
Program Funding
Funds for this pilot program were obtained from the University of Virginia Center for Diabetes Technology, a grant from the School of Architecture Main Street program, and the Department of Medicine. Nutrisystem®D® provided the Nutrisystem®D® program at a reduced cost as stipulated by a research contract signed with that company. The Glytec company, who coordinated the glucommander outpatient program, received a lump sum for their participation which included an extensive educational program and troubleshooting.
Acknowledgements
John Clarke from Glyctec provided invaluable advice about the utilization of the glycommander-outpatient program. Myron Talbert from BioTelcare provided timely assistance in troubleshooting access problems and interpreting the dashboard data presentations. The providers at each of the Federally Funded Community Health Centers interacted very effectively with the protocol team and their help and cooperative is greatly appreciated. Harry Mitchell, Mary Oliveri, and Christian Wakeman coordinated support from the Center for Diabetes Technology. Dr. Andy Basu provided valuable advising in setting up the program and assessing results. The providers and administrative staff at the Federally Qualified Community Health Center clinics assisted all aspects of the study and were indispensable in its ruinning..
References
- Ross S, Benavides-Vaello, Schumann LP, Haberman M (2015) Issues that impact type-2 diabetes self-management in rural communities. Journal of the American Association of Nurse Practitioners 27(11): 653-660.
- Vigersky RA, Fish L, Hogan P, Stewart A, Kutler S, et al. (2014) The clinical endocrinology workforce: Current status and future projections of supply and demand. Journal of Clinical Endocrinology & Metabolism 99(9): 3112-3121.
- Rizza RA. (2003) A model to determine workforce needs for endocrinologists in the United States until 2020. J Clin Endocrinol Metab 88(5): 1979-1987.
- Bell RA, Quandt SA, Arcury TA, Snively BM, Stafford JM, et al. (2005) Primary and specialty medical care among ethnically diverse, older rural adults with type 2 diabetes: The ELDER Diabetes Study. Journal of Rural Health 21(3): 198-205.
- Theresa Capriotti, Tiffany Pearson (2020) Health Disparities in Rural America: Current Challenges and Furutree Solutions Psychiatry Advisor.
- Crowley MJ, Edelman D, McAndrew AT, Kistler S, Danus S, et al. (2016) Practical telemedicine for veterans with persistently poor diabetes control: A randomized pilot trial. Telemedicine Journal & E-Health 22(5): 376-384.
- Nyenwe EA, Ashby S, Tidwell J, Nouer SS, Kitabchi AE (2013) Improving diabetes care via telemedicine: lessons from the Addressing Diabetes in Tennessee (ADT) project. Diabetes Care 34(3): 34.
- Barnett ML, Huskamp HA, Busch AB, Uscher-Pines L, Chaiyachati KH, et al. (2021) Trends in outpatient telemedicine utilization among rural medicare beneficiaries, 2010 to 2019. JAMA health forum 2(10): 213282.
- Gutierrez C (2020) Improving the care of students with diabetes in rural schools utilizing an online diabetes education program for school personnel. Rural and Remote Health 20(1): 5596.
- Marsh Z, Nguyen Y, Teegala Y, Cotter VT (2021) Diabetes management among underserved older adults through telemedicine and community health workers. Journal of the American Association of Nurse Practitioners 34(1): 26-31.
- Zachrison KS, Richard JV, Mehrotra A (2021) Paying for telemedicine in smaller rural hospitals: Extending the technology to those who benefit most. JAMA Health Forum 2(8): 211570.
- Daniel HB, Sulmasy LSJ(2015) Policy recommendations to guide the use of telemedicine in primary care settings: An American college of physicians position paper. Annals of Internal Medicine 163(10): 787-789.
- Wakefield BJ, Koopman RJ, Keplinger LE, Bomar M, Bernt B, et al. (2014) Effect of home telemonitoring on glycemic and blood pressure control in primary care clinic patients with diabetes. Telemedicine Journal & E-Health 20(3): 199-205.
- Karhula T, Vuorinen AL, Raapysjarvi K, Pakanen M, Itkonen P, et al. (2015) Telemonitoring and mobile phone-based health coaching among finnish diabetic and heart disease patients: Randomized controlled trial. Journal of Medical Internet Research 17(6): 153.
- Klonoff DC (2009) Using telemedicine to improve outcomes in diabetes an emerging technology. Journal of Diabetes Science & Technology 3(4): 624-628.
- Klonoff DC (2015) Telemedicine for diabetes: Current and future trends. Journal of Diabetes Science & Technology 10(1) 3-5.
- Klonoff DC (2016) Telemedicine for diabetes: Economic considerations. Journal of Diabetes Science & Technology 10(2): 251-253.
- Cengiz E (2013) Analysis of a remote system to closely monitor glycemia and insulin pump delivery--is this the beginning of a wireless transformation in diabetes management? Journal of Diabetes Science & Technology 7(2): 362-364.
- Bashshur RL, Shannon GW, Smith BR, Woodward MA (2015) The empirical evidence for the telemedicine intervention in diabetes management. Telemedicine Journal & E-Health 21(5): 321-354.
- Zhai YkP, Zhu WjM, Cai YlP, Sun DxB, Zhao JP (2014) Clinical- and cost-effectiveness of telemedicine in type 2 diabetes mellitus: A systematic review and meta-analysis. Medicine 93(28): 312.
- Aikens JE, Zivin K, Trivedi R, Piette JD (2014) Diabetes self-management support using mHealth and enhanced informal caregiving. Journal of Diabetes & its Complications 28(2): 171-176.
- Deborah AG, Heather MY, Charlene CQ (2014) Telehealth remote monitoring. Journal of Diabetes Science and Technology 8(2): 378-89.
- Greenwood DA, Young HM, Quinn CC (2014) Telehealth remote monitoring systematic review: Structured self-monitoring of blood glucose and impact on A1C. Journal of Diabetes Science & Technology 8(2): 378-389.
- Franc S, Daoudi A, Mounier S, Boucherie B, Dardari D, et al. (2011) Telemedicine and diabetes: Achievements and prospects. Diabetes & Metabolism 37(6): 463-476.
- Massey CN, Appel BK, Cherrington AL (2010) Improving diabetes care in ruarl communities: An overview of current initiatives and a call for renewed efforts. Clinical Diabetes 28: 20-27.
- Strawbridge LM, Lloyd JT, Meadow A, Riley GF, Howell BL (2015) Use of medicare's diabetes self-management training benefit. Health Educ Behav 42(4): 530-538.
- Mehrotra AJA, Busch AD, Souza J, Uscher-Pines L , Landon BE (2016) Utilization of telemedicine among rural medicare benificiaries. JAMA 315(18): 2015-2016.
- Crossen S, Raymond J, Neinstein A (2020) Top 10 Tips for successfully implementing a diabetes telehealth program. Diabetes Technology & Therapeutics 22(12): 920-928.
- Quinton JK, Ong MK, Sarkisian C, Casillas A, Vangala S, et al. (2022) The Impact of Telemedicine on quality of care for patients with diabetes after March 2020. Journal of general internal medicine 37(5): 1198-1203.
- Santen RJ, Nass R, Cunningham C, Horton C, Yue W (2023) Intensive, telemedicine-based, self-management program for rural, underserved patients with diabetes mellitus: Re-entry of retired endocrinologists into practice. Journal of Telemedicine & Telecare 29(2): 153-161.
- Churuangsuk C, Hall J, Reynolds A, Griffin SJ, Combet E, et al. (2022) Diets for weight management in adults with type 2 diabetes: An umbrella review of published meta-analyses and systematic review of trials of diets for diabetes remission. Diabetologia 65(1): 14-36.
- Kimberly BB (2022) T2D weight management or glycemic control: The debate continues.
- Lingway I MJ (2022) Experts debate obesity vs glycemic control as primary target for treating type 2 diabetes. Healio.
- Astbury NM, Piernas C, Hartmann-Boyce J, Lapworth S, Aveyard P, et al. (2019) A systematic review and meta-analysis of the effectiveness of meal replacements for weight loss. Obes Rev 20(4): 569-587.
- Foster GD, Wadden TA, Lagrotte CA, Vander Veur SS, Hesson LA, et al. (2013) A randomized comparison of a commercially available portion-controlled weight-loss intervention with a diabetes self-management education program. Nutr Diabetes 3(3): 63.
- Kempf K, Rohling M, Niedermeier K, Gartner B, Martin S (2018) Individualized meal replacement therapy improves clinically relevant long-term glycemic control in poorly controlled type 2 diabetes patients. Nutrients 10(8): 1022.
- Cheskin LJ, Mitchell AM, Jhaveri AD, Mitola AH, Davis LM, et al. (2008) Efficacy of meal replacements versus a standard food-based diet for weight loss in type 2 diabetes: A controlled clinical trial. Diabetes Educator 34(1): 118-127.
- Noronha JC, Nishi SK, Braunstein CR, Khan TA, Blanco Mejia S, et al. (2019) The effect of liquid meal replacements on cardiometabolic risk factors in overweight/obese individuals with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Care 42(5): 767-776.
- (2015) Telcare: sophisticated technology with a personal appraoch.
- Bruce Bode, John Clarke (2017) Glucomander outpatient, a cloud-based management solution, titrated patients to goal in 11 days and sustianed a 2.6% dron in HbA1C over 6 months. International Conference on Advanced Technologies & Treatments for Diabetes, USA.
- Bode B, Clarke JG, Johnson J (2018) Use of decision support software to titrate multiple daily injections yielded sustained A1c reductions after 1 year. Journal of diabetes science and technology 12(1): 124-128.
- Foster GD, Borradaile KE, Vander Veur SS, Leh Shantz K, Dilks RJ, et al. (2009) The effects of a commercially available weight loss program among obese patients with type 2 diabetes: A randomized study. Postgraduate medicine 121(5): 113-118.
- Moreira AM, Marobin R, Escott GM, Rados DV, Silveiro SP (2022) Telephone calls and glycemic control in type 2 diabetes: A PRISMA-compliant systematic review and meta-analysis of randomized clinical trials. Journal of telemedicine and telecare: 1357.
- Santen RJ (2020)"Re-booting" after retirement: Novel approach using telemedicine to solve the work-force gap in diabetes management. Maturitas 133: 68-69.
© 2023 Richard J Santen. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






