- Submissions

Full Text
Trends in Telemedicine & E-health
The Bumpy Fecundation-Placental Site Trophoblastic Tumour
Anubha Bajaj*
Consultant Histopathology, India
*Corresponding author: Anubha Bajaj, Consultant Histopathology, A-1, Ring Road, Rajouri Garden ,New Delhi, India
Submission: March 18, 2022; Published: June 28, 2022

ISSN: 2689-2707 Volume 3 Issue 4
Abstract
Placental site trophoblastic tumour is an infrequently discerned gestational trophoblastic neoplasm emerging from placental implantation site. Tumefaction may emerge subsequent to gestation at term, spontaneous abortion or a molar pregnancy. Of obscure aetiology, the neoplasm arises due to neoplastic transformation of cytotrophoblastic cells wherein transformed cells differentiate towards intermediate trophoblast. Clinically, irregular vaginal bleeding, menorrhagia, abnormal uterine bleeding, amenorrhea or an enlarged uterus are observed. The infiltrative neoplasm is composed of sheets and aggregates of enlarged, spherical or polyhedral, mononuclear intermediate trophoblastic cells imbued with abundant amphophilic, eosinophilic or clear cytoplasm with irregular cytoplasmic perimeter, pleomorphic, enlarged or convoluted, hyperchromatic nuclei and significant nuclear atypia.
Keywords:Gestational neoplasm; Implantation; Intermediate trophoblast
Abbreviations: β-HCG: Beta Human Chorionic Gonadotropin; FIGO: Federation of Gynaecology and Obstetrics; SMA: Smooth Muscle Actin; PLAP: Placental Alkaline Phosphatase; HPL: Human Placental Lactogen; CT: Computerized Tomography; MRI: Magnetic Resonance Imaging; PET: Positron Emission Tomography
Introduction
Placental site trophoblastic tumour is an exceptional gestational trophoblastic neoplasm emerging from placental implantation site. Tumefaction is associated with uncertain malignant potential and an extremely variable clinical course. Initially scripted by Kurman et al in 1976 as “syncytial endometritis”, the infrequent neoplasm with uncharacteristic clinical representation may be challenging to discern [1]. Placental site trophoblastic tumour is additionally designated as Sertoli cell pseudo-tumour, atypical choriocarcinoma, syncytioma, chorio-epitheliosis or trophoblastic pseudo-tumour. Tumefaction is engendered by neoplastic proliferation of intermediate trophoblastic cells although a lack of cyto-trophoblastic cells and chorionic villi is exemplified. The condition may be misinterpreted as a missed abortion
Disease Characteristics
The neoplasm is commonly discerned in uterine segments of young women within the reproductive age group. However, post-menopausal women may depict placental site trophoblastic tumour. Mean age of disease discernment is up to 33 years although the neoplasm may emerge up to sixth decade. The neoplasm is generally discerned within uterine corpus or lower uterine segment. Fallopian tubes and ovaries may be exceptionally implicated [2,3]. Majority (85%) of neoplasms exhibit a female antecedent gestation. Serum Beta Human Chorionic Gonadotropin (β-HCG) is detectable in a majority (80%) of instances [2,3]. The neoplasm may emerge subsequent to gestational events as a term pregnancy, spontaneous abortion or pregnancy with hydatidiform mole. Tumefaction following full term pregnancy exhibits a median latent duration of 12 months to 18 months although may emerge within two decades [2,3]. Mean interval between a gestational event and detection of placental site trophoblastic tumour may vary from several weeks to up to 15 years. Placental site trophoblastic tumour may appear concurrent to twin pregnancy [2,3]. Additionally, the neoplasm may emerge within a non-pregnant uterus, ovary of iso-sexual precocious puberty, male subjects and as a subtype of non-seminomatous germ cell tumour [2,3]. Of obscure aetiology, the neoplasm may arise due to neoplastic transformation of cytotrophoblastic cells wherein the transformed cells differentiate towards intermediate trophoblast of the implantation site. Alternatively, duplication of paternal X chromosome may engender an anomalous genetic saturation with consequent trophoblastic proliferation [2,3]. Placental site trophoblastic tumour configures around 1% to 2% of gestational trophoblastic neoplasia and may convert into a choriocarcinoma.
Majority (>80%) of neoplasms configure to stage I of International Federation of Gynaecology and Obstetrics (FIGO) staging. Tumour reoccurrence or distant metastasis ensues in nearly 30% subjects and tumour associated mortality is observed in roughly 25% subjects [2,3]. An estimated 10% instances of placental site trophoblastic tumour exhibit distant metastasis which predominantly emerges within the vagina, pulmonary parenchyma or brain. The neoplasm delineates a paternally derived X chromosome and absence of Y chromosome [2,3].
Clinical Elucidation
Non-specific or Non-characteristic clinical representation of placental site trophoblastic tumour contributes to challenging disease discernment [4,5]. Placental site trophoblastic tumour represents with heterogeneous clinical manifestations as irregular vaginal bleeding, menorrhagia, abnormal uterine bleeding, amenorrhea or enlarged uterus. Mild cervical erosion and hypertrophy may be observed. Exceptionally, nephrotic syndrome, virilization, sepsis or erythrocytosis may ensue [4,5]. Upon initial discernment, placental site trophoblastic tumour is usually confined to uterine segments. Lesions incriminating the fallopian tube possibly occur following tubal pregnancy and engender significant internal haemorrhage [4,5]. Besides, tumour metastasis may appear as initial disease manifestation. The neoplasm can represent as fulminant metastatic disease which is unresponsive to conventional therapeutic modalities [4,5].
Histological Elucidation
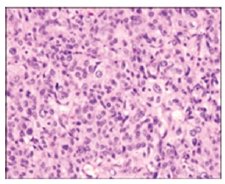
Upon gross examination, a well circumscribed, nodular or polypoid tumefaction of up to 10 centimetres magnitude may protrude into the uterine cavity or predominantly infiltrate the myometrium. Deep-seated myometrial invasion may emerge in around 50% instances. Frequently, tumour invasion extends towards uterine serosa whereas infiltration of adnexal structures or broad ligament is exceptional [5,6]. Tumefaction appears as purple-blue, poorly circumscribed lesion infiltrating the uterine myometrium with accompanying haemorrhage and tumour necrosis. Cut surface exhibits a solid, fleshy, white, tan or light yellow neoplasm enunciating focal haemorrhage or necrosis [5,6]. Placental site trophoblastic tumour enunciates an infiltrative pattern of tumour evolution and is characteristically configured of mononuclear, intermediate trophoblastic cells admixed with occasional, multinucleated intermediate trophoblastic cells and giant cells infiltrating the myometrium and vascular articulations. Proliferation of Sertoli cells along with an absence of chorionic villi may be observed [5,6] (Figures 1 & 2). Tumefaction is composed of sheets and aggregates of enlarged, spherical or polyhedral, mononuclear derived from implantation site intermediate trophoblastic cells [5,6]; (Figures 3 & 4). Tumour cells are imbued with abundant amphophilic, eosinophilic or clear cytoplasm, an irregular cytoplasmic perimeter, pleomorphic, enlarged or convoluted, hyperchromatic nuclei with significant nuclear atypia [5,6]; (Figures 5 & 6). Characteristically, vascular invasion is discerned wherein the tumour cells supplant myometrial vascular walls.
Figure 1:Placental site trophoblastic tumour depicting intermediate trophoblastic cells with abundant eosinophilic cytoplasm, few multinucleated cells and focal necrosis.
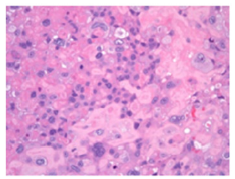
Figure 2:Placental site trophoblastic tumour delineating intermediate trophoblastic cells infiltrating the myometrium.
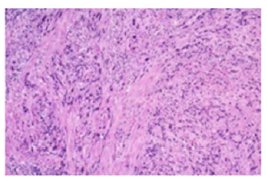
Figure 3:Placental site trophoblastic tumour demonstrating intermediate trophoblastic cells with cellular and nuclear atypia, focal necrosis and myometrial invasion.
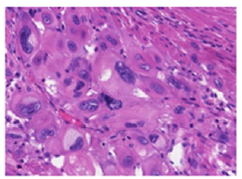
Figure 4:Placental site trophoblastic tumour exhibiting intermediate trophoblastic cells invading vascular articulations and myometrial cells.

Figure 5:Placental site trophoblastic tumour enunciating extensive myometrial invasion by intermediate trophoblastic cells admixed with focal haemorrhage and vascular invasion.
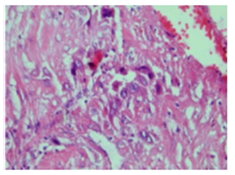
Figure 6:Placental site trophoblastic tumour exemplifying cords and fascicles of intermediate trophoblastic cells with abundant eosinophilic cytoplasm and uniform nuclei.
The unique, transformed vascular configurations are pathognomonic of placental site trophoblastic tumour [5,6]. Upon microscopy, proliferation of medium-sized, spindleshaped or polyhedral mononuclear cells akin to intermediate trophoblastic cells are observed. Multinucleated cells implantation site trophoblastic cells may appear disseminated and admixed with mononuclear intermediate trophoblastic cells. Decidua or an arias-stella reaction may be discerned within the adjacent, uninvolved endometrium. Cyto-trophoblastic tissue or chorionic villi are usually absent [5,6]; (Figures 7 & 8). Aggregates and confluent sheets of tumour cells are intermingled with singularly dispersed or cords and nests of peripherally disseminated intermediate trophoblastic cells. Typically, tumour cells infiltrate the myometrium and segregate individual skeletal muscle fibres and fibre groups [5,6]. Majority of neoplasms exhibit a minimal mitosis of up to 2 per 10 high power fields. However, neoplasms of enhanced tumour grade may exemplify an elevated mitotic activity beyond >5 per 10 high power fields. Foci of tumour-induced haemorrhage and necrosis may be delineated [5,6]. Occurrence of tumour necrosis, nuclear atypia and frequent mitosis are indicative of a high-grade placental site trophoblastic tumour. Tumour cells may demonstrate nuclear atypia along with focal infiltration of myometrium and mitosis exceeding 5 per 10 high power fields. Focal chronic endometritis and chronic cervicitis may concur [5,6]. Immunohistochemistry The neoplasm is intensely immune reactive to human placental lactogen (HPL), CD146, CD10, GATA3, PDL1, hydroxy-delta-5-steroid dehydrogenase 3 beta and steroid deltaisomerase 1 (HSD3B1), cytokeratin, MUC4 and Smooth Muscle Actin (SMA). Cells minimally immune reactive to beta human chorionic gonadotropin (β-HCG) appear disseminated between intermediate trophoblastic cells and multinucleated giant cells. Tumour cells are focally immune reactive to inhibin. Immune reactivity to prolactin or gonadotropins may be delineated [6,7].
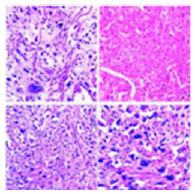
Figure 7:Placental site trophoblastic tumour demonstrating aggregates and nests of intermediate trophoblastic cells with amphophilic cytoplasm and minimal atypia invading the myometrial fascicles.

Figure 8:Placental site trophoblastic tumour demonstrating clusters of intermediate trophoblastic cells with moderate cellular and nuclear invading the myometrium.
Differential Diagnosis
Placental site trophoblastic tumour requires a segregation from
conditions such as
• Exaggerated placental site which displays an exuberant
infiltration of implantation site intermediate trophoblastic
cells. The intermediate trophoblastic cells are demarcated by fascicles of hyaline tissue, demonstrate an absence of mitosis
and are intermingled with innumerable multinucleated
trophoblastic cells, decidua and chorionic villi [7,8].
• Squamous cell carcinoma of cervix is composed of
tumour cells which configure and infiltrate surrounding
stroma as irregular, anastomosing tumour cell nests or
singular cells. Enveloping stroma can be desmoplastic or
invaded with inflammatory cells. Foci of stromal dehiscence
or desmoplastic reaction can be observed. Superficial stromal
invasion or lymphoid and vascular invasion may be delineated.
Tumour grading is contingent to features such as nuclear
pleomorphism, nucleolar magnitude, mitotic activity and
tumour cell necrosis. Keratin pearls, abundant keratohyaline
granules and intercellular bridges may be exemplified. Tumour
cells depict enlarged, hyperchromatic nuclei, coarse chromatin
and inconspicuous nucleoli. The neoplasm is immune reactive
to Human Papilloma Virus (HPV), cytokeratin 5, cytokeratin 6
and p16. Ki67 proliferation index is significantly enhanced. The
neoplasm is immune non-reactive to cytokeratin 18, Human
Placental Lactogen (HPL), CD146, MUC4 and HSD3B1 [7,8].
• Epithelioid trophoblastic tumour is a nodular, expansible,
well circumscribed neoplasm exhibiting focal, peripheral
tumour infiltration. Mononuclear, uniform tumour cells
are configured in nests and cords. Tumour cell nests are
admixed with an eosinophilic, fibrillary, hyaline-like substance
composed of type IV collagen along with onco-foetal and adult
subtypes of fibronectin. Cells of chorionic- type, intermediate
trophoblastic tissue exhibit moderate, eosinophilic to clear
cytoplasm imbued with glycogen, spherical nuclei, miniature,
distinctive nucleoli and distinct cellular membranes. Tumour
calcification is frequent. Extensive geographic necrosis may be
discerned. Circumscribing stromal cells appear decidua-like.
Tumour cells are immune reactive to p63, Placental Alkaline
Phosphatase (PLAP) and inhibin [7,8].
• Choriocarcinoma is an extremely exceptional, malignant
placental neoplasm depicting extensive proliferation of
trophoblastic cells with accompanying focal necrosis and
cellular and nuclear atypia. The controversial neoplasm may
arise within a chorangioma or may be composed of an admixture
of chorangioma and choriocarcinoma. The neoplasm is
comprised of a trimorphic proliferation of syncytiotrophoblast,
cytotrophoblast and intermediate trophoblast. Tumefaction
is associated with possible maternal and neonatal distant
metastases. Tumour cells are intensely and diffusely immune
reactive to serum β-HCG. Ki67 proliferation index is significantly
enhanced to beyond >40% [7,8].
• Poorly differentiated carcinoma is a neoplasm which may
depict glandular differentiation or variable foci of epithelial
differentiation as squamous epithelium or fallopian tubespecific
ciliated columnar epithelium [7,8].
• Epithelioid smooth muscle tumour characteristically
displays a fascicular pattern of tumour evolution. The
neoplasm is composed of epithelioid cells with uniform
nuclei and abundant, eosinophilic cytoplasm. Tumefaction is
immune reactive to Smooth Muscle Actin (SMA) and desmin.
Tumefaction is immune non-reactive to Human Placental
Lactogen (HPL), CD146 and HSV3B1 [7,8].
• Metastatic deposits of malignant melanoma are
composed of tumour cells imbued with fine granules or dusty
melanin pigment. Tumour cell nuclei are imbued with enlarged
nucleoli. Tumour cells are diffusely immune reactive to human
melanoma black 45 (HMB45), melan A and S100 protein [7,8].
Investigative Assay
Adequate tumour determination is challenging and necessitates concurrent evaluation of serum beta human chorionic gonadotropin (β-HCG) levels, radiographic assay and histological assessment with pertinent immunohistochemistry [9,10]. Plain radiography and Computerized Tomography (CT) of the thoracic cavity may exhibit multiple, metastatic nodules confined to pulmonary parenchyma. Abdominal and pelvic viscera may appear unremarkable. Upon ultrasonography, a solid tumour mass appears confined within the endometrial cavity or myometrium. Uterine cavity is enlarged with a heterogeneous echotexture. Also, echogenic foci, cystic areas and focal haemorrhage may be discerned [9,10]. Ultrasonography exhibits a heterogeneous, irregular, ill-defined echo of variable magnitude confined to diverse uterine walls. The uterus may depict a honeycomb echo, fresh, focal haemorrhage and regression of endometrial layer. Besides, ultrasonography may indicate the occurrence of “Sertoli cell tumour”. Alternatively, a hypoechoic, ovarian tumefaction is discerned with ultrasonography [9,10]. Colour doppler exhibits prominent vascular articulations. Serum beta human chorionic gonadotropin (β-HCG) levels are nonconcordant and non-predictive with tumour burden, disease progression or potential malignant metamorphosis and may be normal or moderately elevated. Serum gonadotropin levels may be normal or elevated. Regular monitoring with adequate clinical examination, evaluation of serum β-HCG levels, ultrasonography of pelvic and abdominal viscera and Computerized Tomography (CT) of the thoracic cavity is mandated in order to exclude localized or systemic tumour reoccurrence [9,10].
Upon Magnetic Resonance Imaging (MRI), a distorted junctional zone ensues on account of an endometrial or myometrial tumefaction. Upon T1 weighted imaging, an isointense tumefaction or a normal myometrium is observed. Upon T2 weighted imaging an isointense or minimally hyper-intense, myometrial signal intensity is delineated [9,10]. Besides, a diffusely heterogeneous uterine pattern is associated with decimated zonal anatomy [9,10]. Localized uterine resection may be preceded by ultrasonography, Magnetic Resonance Imaging (MRI) or Positron Emission tomography (PET) in order to identify site of residual neoplasm [9,10].
Therapeutic Options
Surgical extermination is necessitated for resection of neoplasm and adjacent, infiltrated viscera [10,11]. Majority of non-metastatic placental site trophoblastic tumours may be appropriately subjected to total abdominal hysterectomy along with or in the absence of bilateral salpingo-oophorectomy. Nevertheless, a significant proportion of neoplasms mandate aggressive surgical manoeuvers along with adjuvant chemotherapy or radiation [10,11]. Exploratory laparotomy, total abdominal hysterectomy and oophorectomy may be adopted as cogent, preferential surgical procedures and are recommended for treating placental site trophoblastic tumour [10,11]. Total abdominal hysterectomy along with preservation of ovaries may be employed. Majority of tumefaction are alleviated by simple hysterectomy. Conservative surgical manoeuvers such as dilatation and curettage or localized uterine resection are recommended for preserving fertility [10,11]. Generally, the neoplasm is resistant to adjuvant chemotherapy which may be employed for treating neoplasms following surgical intervention and appear associated with inferior prognosis [10,11]. Nevertheless, combination therapy with agents such as methotrexate, actinomycin and cyclophosphamide may be beneficial. Combination chemotherapy is associated with significant therapeutic response and extended tumour remission, features beneficial with repetitive or metastatic lesions. However, a comprehensive response and tumour alleviation is infrequent [10,11]. Total abdominal hysterectomy followed by adjuvant chemotherapy within one week is optimal in decimating proportionate tumour reoccurrence. EMA/EP chemotherapy regimen composed of etoposide, cisplatin, methotrexate and dactinomycin or EMA/CO regimen comprised of etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine appear efficacious in treating metastatic or reoccurring placental site trophoblastic tumour [10,11].
Second-look surgical procedures along with multiple courses of combined chemotherapy may emerge as a pre-requisite for disease remission [10,11]. Factors contributing to an inferior therapeutic outcome are extra-uterine metastases delayed emergence of neoplasm beyond > two years following a preceding gestational event incriminated females beyond forty years enlarged tumefaction with tumour cells exhibiting features of enhanced histological grade such as extensive tumour necrosis, nuclear atypia, tumour cells with clear cytoplasm, deep-seated myometrial invasion, tumour necrosis, mitotic activity exceeding >5 per 10 high power fields and an elevated Ki-67 proliferation index, usually below <30% [10,11]. Prognostic outcomes are variable and inconsistent. Tumour confined to the uterus and disease emergence within 2 years of antecedent gestational event exhibit a superior prognosis. In contrast, extra-uterine disease dissemination is a significant, unfavourable prognostic factor followed by duration from preceding gestational event exceeding >2 years. Mitotic count in excess of >5 per 10 high power fields depicts an inferior survival [10,11]. Delayed emergence of the neoplasm following a gestational event is associated with an inferior prognostic outcome. Nevertheless, an advanced tumour stage, duration around ≥48 months following an antecedent gestational event, tumour emergence beyond >40 years and tumour cells with clear cytoplasm may independently concur an unfavourable outcome [10,11]. Also, distant tumour metastasis to vital organs such as brain contributes to tumour associated mortality irrespective of cogent therapeutic interventions. Metastatic neoplasms are associated with significant mortality in spite of intensive, multimodal chemotherapy [10,11].
Conclusion
Placental site trophoblastic tumour requires a segregation from an exaggerated placental site, squamous cell carcinoma of cervix, epithelioid trophoblastic tumour, choriocarcinoma, poorly differentiated carcinoma, epithelioid smooth muscle tumour or metastatic deposits of malignant melanoma. Concurrent evaluation of serum beta human chorionic gonadotropin (β-HCG) levels, radiographic assay and histological assessment with pertinent immunohistochemistry is pertinent for adequate tumour discernment. Total abdominal hysterectomy and oophorectomy is a cogent, preferential, surgical manoeuver for treating the neoplasm.
References
- Kurman RJ, Scully RE, Norris HJ (1976) Trophoblastic pseudo-tumour of the uterus. An exaggerated form of "syncytial endometritis" simulating a malignant tumor. Cancer 38(3): 1214-1226.
- Hancock BW, Tidy J (2021) Placental site trophoblastic tumour and epithelioid trophoblastic tumour. Best Pract Res Clin Obstet Gynaecol 74: 131-148.
- Peixinho C, Almeida A, Carla B, Pires MC (2021) Placental site trophoblastic tumour: Five challenges of patient clinical management. BMJ Case Rep 14(1).
- Behtash N, Ghaemmaghami F, Hasanzadeh M (2005) Long term remission of metastatic Placental Site Trophoblastic Tumour (PSTT): Case report and review of literature. World J Surg Oncol 3: 1-4.
- Shih IeM (2007) Gestational trophoblastic neoplasia-pathogenesis and potential therapeutic targets. Lancet Oncol 8(7): 642-650.
- Huang F, Zheng W, Liang Q, Yin T (2013) Diagnosis and treatment of placental site trophoblastic tumour. Int J Clin Exp Pathol 6(7): 1448-1451.
- Chen BJ, Cheng CJ, Chen WY (2013) Transformation of a post-caesarean section placental site nodule into a coexisting epithelioid trophoblastic tumour and placental site trophoblastic tumour: A case report. Diagn Pathol 8: 85.
- Ou-Yang RJ, Hui P, Yang XJ, Zynger DL (2010) Expression of glypican 3 in placental site trophoblastic tumour. Diagn Pathol 5: 64.
- Lurain JR (2011) Gestational trophoblastic disease II: Classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol 204(1): 11-18.
- Baergen RN, Rutgers JL, Young RH, Osann K, Scully RE (2006) Placental site trophoblastic tumour: A study of 55 cases and review of the literature emphasizing factors of prognostic significance. Gynecol Oncol 100(3): 511-520.
- Hyman DM, Bakios L, Gualtiere G, Carr C, Grisham RN, et al. (2013) Placental site trophoblastic tumour: Analysis of presentation, treatment and outcome. Gynecol Oncol 129(1): 58-62.
© 2022 Anubha Bajaj. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






