- Submissions

Full Text
Trends in Telemedicine & E-health
The Myth of Significant Fluid Loss in Acute Pneumonia
Klepikov I*
Department of Pediatric Surgeon, USA
*Corresponding author: Klepikov I, Department of Pediatric Surgeon, USA
Submission: August 20, 2021; Published: September 08, 2021

ISSN: 2689-2707 Volume 3 Issue 1
Introduction
Modern ideas about the nature of Acute Pneumonia (AP), formed under the influence
of long-term beliefs in the exceptional role and therapeutic indispensability of antibiotics,
have left many fundamental laws of medical science without the necessary attention. The
search for a solution to the problem of this disease cannot be successful without a revision of
the conceptual views that determine both the search directions and subsequent therapeutic
efforts.
Currently, almost all urgently hospitalized patients immediately get access to the venous
bed and begin to receive an infusion of solutions. This priority of this procedure is due not
only to the need to have the most effective way of administering medications, but also to
compensate for the loss of fluid, which in acute diseases has many reasons for this. Further
recommendations for the correction of water-electrolyte and volume losses and the choice of
the infusion rate are determined by the general criteria for their diagnosis in accordance with
the parameters of the large circle of blood circulation. Considering AP, first of all, as a result of
infection and not focusing on the localization of the process, modern medicine does not make
exceptions in this therapeutic direction for patients with inflammation of the lung tissue.
For many years, fever and tachypnea were considered the main causes of fluid deficiency
in patients with AP [1]. But the role of these factors in the occurrence of inconspicuous
losses is hardly worthy of comparison with the consequences of homeostasis disorders that
accompany such diseases as, for example, enterocolitis or peritonitis, when the body really
loses large volumes of fluid, and these losses are quite noticeable and can be assessed both
quantitatively and qualitatively.
Despite such a significant difference between demonstrative and hidden losses, the
recommendations regarding the volume and speed of infusions in severe patients with
inflammatory diseases are the same, regardless of the location of the primary focus [2-5].
From my point of view, the lack of liquid in the AP, which occurs in a short time as a result
of evaporation, is clearly exaggerated. Practical medicine does not have precise methods for
determining the losses expected as a result of perspiration. At the same time, one of the main
reasons for the appointment of infusion therapy for AP is the tendency of these patients to
hypotension. It is this sign that serves as a guideline for intravenous infusions, since the next
recommendation after the start of bolus infusions, which often do not achieve the expected
effect, is the introduction of vasopressors to these patients.
The idea of the causes of the severity of clinical manifestations of AP, which today is
based primarily on the characteristics of the pathogen, changes significantly if we recall the
fundamental foundations of inflammation in general and lung tissue in particular. In modern
publications, the mechanism accompanying inflammation in the lungs is not given due
attention, so the most severe cases of AP that require intensive treatment are not analyzed
as a separate group. The general material for the analysis of such conditions usually includes
information about various diseases, in which patients with lung tissue inflammation account
for up to 40-50% [3]. The combination of diseases with diametrically opposite pathogenetic
mechanisms is a very serious misconception in such analytical work.
In this regard, first of all, it is necessary to remember that the
basis of the inflammatory transformation of tissues in the affected
area is the indispensable development of a consistent reaction of
blood vessels with impaired blood flow and increased permeability
of their walls, as well as the mandatory accompaniment of these
changes with five classic signs of inflammation (heat, pain, redness,
swelling and loss of function). The last sign, a violation of the
function of the affected organ, plays a leading role in the clinical
manifestations of the disease.
But the main feature of the topic under discussion is the fact that
AP is the only representative of inflammatory processes occurring
in the small circle of blood circulation, unlike all other nosologies
localized in the large circle. The inverse proportion of the functional
state between the two circulatory circles with their inseparable
anatomical and functional connection and interdependence
underlies the differences in the pathological mechanisms
accompanying the different localization of the primary focus of
inflammation. In this regard, the interpretation of the pathogenesis
of AP by analogy with other forms of acute inflammation can in no
way have the same scenario and monitoring of emerging functional
disorders should have a different understanding.
In healthy people, the blood pressure in the pulmonary vessels
is always several times lower than in the arteries of the great circle
[6,7]. This difference is maintained automatically, allowing the two
halves of the heart to synchronously perform their functions and
direct equal volumes of blood to completely incomparable vascular
systems in anatomical parameters. Maintaining this balance is a
vital condition for the body since possible shifts of these functional
parallels lead to conditions incompatible with life.
The appearance of a focus of acute inflammation in the vessels
of the small circle is a disaster for the body and a cause that disrupts
the balance between the two halves of the circulatory system. The
localization of this zone not only creates a physical obstacle to the
main blood flow, which is ejected by the right half of the heart (Figure
1), but also is a source of reflex spasm of the pulmonary vessels [8-
11]. The pressure in the vessels of the small circle increases, and
its throughput decreases, creating an excess of venous return. To
correct this situation and avoid asynchronous operation of the
cardiovascular system, the body changes the parameters of the
large circle of blood circulation, reducing the pressure in it and
increasing its volume for a sudden “excess” of circulating blood [8].
Figure 1: Schematic representation of the human circulatory system.
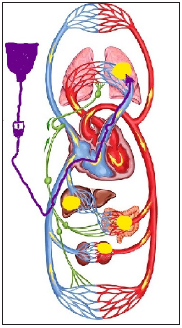
Explanation 1
a) The comparative value of foci of acute inflammation
(yellow fields) for different departments and volumes of blood flow,
depending on the possible localization.
b) The initial route of intravenous administration of
solutions (dark purple arrow).
Explanation 2
The mechanism of hypotension in the large circle of blood
circulation in AP as a result of damage to the pulmonary vessels is
especially manifested in the aggressive development of the process.
In this situation, it is not difficult to imagine the role played by
infusions that increase venous return and additional blood flow to
the focus of inflammation.
However, the effect of infusion therapy on the development of
AP is a much more complex process than its visual version shown
in the figure. The need to clarify the role of infusion therapy in the
dynamics of the development of lung inflammation arose in our
work many years ago, when the most aggressive bacterial forms of
AP began to be purposefully hospitalized in our department during
the initial period of the disease. The concentration of a large number
of such patients was accompanied by the rapid development of
pleural complications and high mortality in them and resembled the current situation with the isolation of COVID-19 pneumonia.
One of our typical examples of the dynamics of the disease is the
following observation.
A 2-year-old girl was taken to the clinic with abdominal pain
and shortness of breath 12 hours after their appearance. According
to her medical history, the child was healthy, but in the last few
days she had a mild respiratory syndrome with a runny nose and a
cough without fever. Upon admission to the clinic, the patient was
diagnosed with AP (Figure 2). Intensive treatment was immediately
started, including intravenous administration of two antibiotics
and intravenous fluids up to 30ml/kg/hour for 2 hours, followed
by a decrease in the infusion rate to 10ml/kg/hour. Despite the
treatment, the child’s condition did not improve, and a control
radiograph was diagnosed with pyopneumothorax 36 hours after
hospitalization (Figure 3). The pus obtained from the pleural cavity
during drainage was subjected to bacteriological and microscopic
examination, but no microflora was found in it.
Figure 2: X-ray photograph of 2 years girl 12 hours after the first signs of AP with abdominal pain syndrome were discovered. There is homogeneous shading in a middle-right pulmonary field.
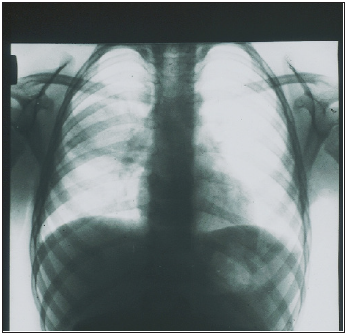
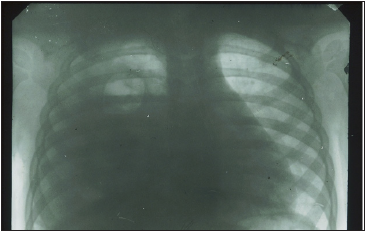
Figure 3: X-ray of the same patient, 36 hours after the start of inpatient treatment. There is an intense uniform darkening of almost the entire right hemithorax with a displacement of the mediastinum to the left, as well as a cavity with a fluid level in the upper pulmonary field.
The presented observation cannot be an absolute proof of
the negative effect of infusion therapy on the dynamics of the
inflammatory process in the lung. The results of the observed
transformation in the area of inflammation only allow us to assume
such a dependence and draw appropriate conclusions on an
empirical basis. Therefore, in order to find additional arguments in
favor of such an assumption, which cannot be obtained in clinical
conditions, animal experiments were conducted. The volume of the
description of experimental studies does not allow us to present
them in the framework of a journal article. However, if it is necessary
to obtain this information, it can be found in available sources
[12,13]. Only the section of the study that is directly relevant to the
issue under discussion is given here.
First of all, a model of the bronchogenic form of AP was created.
At the same time, in order to reduce the charismatic etiology of the
disease and to assess the significance of other factors, cultures
of microbes that are usually not considered as pathogens of AP
were used. The choice was made in favor of E Escherichia coli and
Staphylococcus epidermidis. When a statistically reliable stable
production of the AP model was obtained, in the final series of
experiments, intravenous infusions of solutions were administered
to rabbits during the occurrence of inflammation in the lungs. The
volume of infusions was 30ml/kg/hour and was performed once a
day for 3 days. In addition, in 6 cases, the addition of a methylene
blue dye to the infusion solution was used. This technique was
borrowed from the experiments of V. Mеnkin, who discovered the
permeability factor [14].
The results obtained after euthanasia of animals on the fourth
day of the experiment showed the following. Reproduction of the AP
model without subsequent intravenous infusions was accompanied
by the development of local inflammation of the lung tissue with a
slight pleural reaction in some cases. Intravenous infusions in all
cases were accompanied by the development of parapneumonic
pleurisy. In two cases, pyopneumothorax was detected, the cause
of which was small foci of destruction in the lung tissue. After
infusions with the addition of dye, weakly colored lung tissues were
found along the periphery of the inflammatory focus (Figure 4).
Thus, the results of the experiments allowed us to obtain
additional and undoubted confirmation of the negative role of
intravenous infusions in AP. The use of the dye demonstrated a
visual effect of the spread of inflammatory infiltration in the lung
tissue, which is a consequence of increased blood flow to the area
with increased vascular permeability. In addition, it should be
emphasized that, despite the strict repetition of the experimental
conditions in each specific case, the final results represented a
number of different variants of pathology.
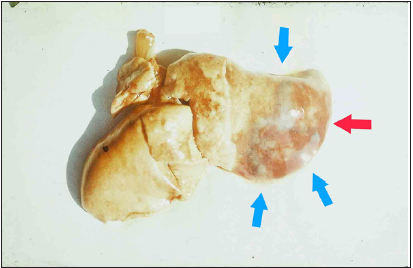
Figure 4: Macro-preparation of the lung (experiment, series 4b). Massive focus of the inflammation in a pulmonary surface (red arrow), surrounded by the additional sections of infiltration with blue shading (blue arrows). Explanations in the text.
The presented information allows us to analyze the reasons for
the continued growth of pleural empyema in patients with AP from
a different angle, even in regions with advanced healthcare systems
[15,16]. Such an analysis will allow us to evaluate one of the
pathogenetic mechanisms of AP and understand why communityacquired
pneumonia occurs with parapneumonic effusions in 20-
50% of cases, and pleural empyema often turns out to be sterile in
microbiological studies [17].
Today, the search for effective methods of treating AP is based
on the concept of the disease, which has developed under the
influence of long-term and insufficiently critical use of antibiotics.
The dominant idea of the leading role of pathogens in the
development of the disease and the oblivion of the fundamental
laws of life of biological objects form the narrowly focused efforts of
this search work and lead to problematic conclusions. This message
is intended to draw attention to only one of the existing conceptual
misconceptions, without correcting which the further success of
treatment of this category of patients looks, to put it mildly, very
doubtful.
References
- Berlin DA, Gulick RM, Martinez FJ (2020) Severe covid-19. N Engl J Med 383: 2451-2460.
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, et al. (2017) Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Medicine 43(3): 304-377.
- Ceccato A, Torres A (2018) Sepsis and community-acquired pneumonia. Ann Res Hosp 2(7).
- Alhazzani W, Møller MH, Arabi YA, Loeb M, Gong MN, et al. (2020) Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med 46(5): 854-887.
- Lim TK, Chew MY (2018) Management of severe community acquired pneumonia in the emergency department. J Emerg Crit Care Med 2(1): 2.
- https://en.wikipedia.org/wiki/Pulmonary_artery#Pulmonary_artery_pressure
- (2017) Normal hemodynamic parameters - adult. Edwards Lifesciences LLC, California, USA.
- Schwiegk H (1935) Der Lungenentlastungsreflex. Pflügers Arch Ges Physiol 236: 206-219.
- Klepikov I (2017) The meaning of pulmonary reflexes in the pathogenesis of acute pneumonia. Intern Med 7: 232.
- Klepikov I (2018) Acute pneumonia is more cardiovascular than respiratory disaster. J Emerg Med Care 1(1): 105.
- Thillai M, Patvardhan C, Swietlik EM, McLellan T, Backer JD, et al. (2021) Functional respiratory imaging identifies redistribution of pulmonary blood flow in patients with COVID-19. Thorax 76(2): 182-184.
- Klepikov I (2020) Acute pneumonia. New doctrine and first treatment results. Lambert Academic, California, USA.
- Klepikov I (2021) How many pneumonias exist in nature? Generis Publishing, Moldova, Europe.
- Valy M (1940) Dynamics of inflammation. Macmillan, New York, USA.
- Arnold DT, Hamilton FW, Morris TT, Payne R, Maskell NA (2019) S12 the changes in incidence and management of pleural empyema in England over the last decade. Thorax 74: A9-A10.
- Bobbio A, Bouam S, Frenkiel J, Zarca K, Fourne L, et al. (2021) Epidemiology and prognostic factors of pleural empyema. Thorax.
- Hassan M, Patel S, Sadaka AS, Bedawi EO, Corcoran JP, et al. (2021) Recent insights into the management of pleural infection. International Journal of General Medicine 14: 3415-3429.
© 2021 Klepikov I. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






