- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
The Effect of Guanidine on Colour Fastness of Acid Dyes Applied to Polyamide Fibres
David M Lewis1* and Megan A Davenport-Connolly2
1University of Leeds, School of Chemistry, UK
2Royal Academy of Engineering, 3 Carlton House Terrace, UK
*Corresponding author:David M Lewis, School of Chemistry, University of Leeds, Leeds LS2 9JT, UK
Submission: March 12, 2024; Published: March 22, 2024

ISSN 2578-0271 Volume9 Issue5
Abstract
Guanidine is a very strong base and even under alkaline conditions above pH 11 is present in aqueous
conditions as the protonated guanidinium cation. This property does not seem to have been exploited for
fixing sulfonated dyes on polyamide substrates.
This study demonstrates that guanidine chloride can be used to after-treat acid dyeings on nylon 6,6 to
impart very good wash-fastness properties. The after-treatment can be carried out effectively at pH 10
for 15 minutes.
Keywords:Alkaline; Guanidinium cation; Polyamide substrates
Introduction
Guanidine [1] is a complex material which is strongly basic (pKa 13.6). Even when dissolved in water guanidine is protonated to form the guanidinium ion. The following structures are relevant (Scheme 1).
Scheme 1:Structures of guanidine and guanidinium chloride.
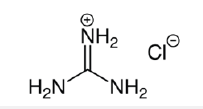
Hydrochloride Salt
Guanidine is commonly available in the pharmaceutical field as guanidinium hydrochloride; this salt is very soluble in water giving pH 4.5, however many other salts are very insoluble in water. It is perhaps surprising that the guanidinium cation does not seem to have been used in the manufacture of pigments through precipitation of sulfonated dyes. This short communication investigates the use of a guanidinium chloride (GC) aftertreatment to improve the wash-fastness properties of acid dyes applied to nylon 6,6. Nylon 6,6 is a polyamide fibre having the following structure: (Scheme 2).
Scheme 2:Structure of nylon 6,6.
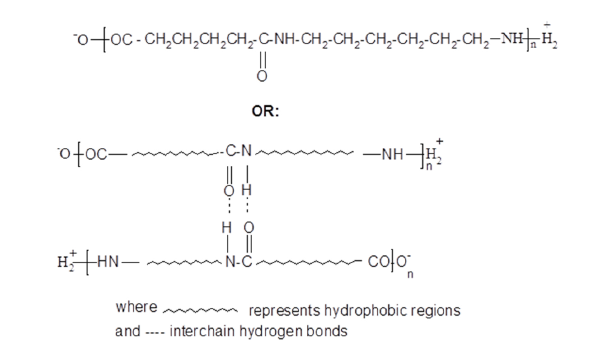
Commercially available nylon 6,6 fibre contains 0.036 moles of primary amino groups and 0.090 moles of carboxylic acid groups situated at the ends of the polyamide chains. At ambient temperatures, the nylon fibre thus exists in the following ionic states according to the pH of the surrounding aqueous solution: (Scheme 3).
Scheme 3:Overall fibre charge when dyeing nylon vs pH.
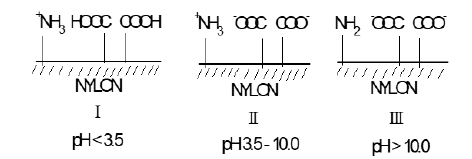
The above indicates that to dye nylon with sulfonated acid dyes mildly acidic conditions have to be used to protonate the carboxylate and amino residues [2,3]. At pH 10.5 it is clear that the overall charge on nylon is negative (-COO- groups predominate) and thus the cationic guanidinium residue in the GC molecule will be substantive to the fibre.
Experimental
Materials
Nylon 6,6 fabric was supplied by Invista Ltd (UK) - standard full-dull T6200 with an amino end group content of 60-65mmol/ kg fibre.
CI Acid Red 33, CI Acid Black 1, CI Acid Orange 7 dye samples were supplied by the Department of Colour Science, University of Leeds.
Chemicals were supplied by Aldrich (UK).
Methods
Dyeing was carried out by the long-liquor ‘exhaustion’ dyeing procedure using a Vistacolor laboratory dyeing machine (Zeltex, Switzerland).
The standard ISO 3 wash-test (5gdm-3 sodium carbonate, 5gdm3 soap at 60C for 15 mins) was carried out on the selected dyed samples.
Dyeing efficiency before and after various post-treatments was calculated by measuring the colour intensity of dyed samples using the Datacolour Spectra flash 500 dual beam reflectance spectrophotometer (Datacolour, UK), which gives reflectance values at wavelengths between 400 and 700nm. From these values of reflectance, Kubelka-Munk values (K/S) were determined: K/S = (1-R)2/2R
K and S are the absorption coefficient and scattering coefficient of a colorant at a given wavelength, R is the fraction of reflectance of substrate at the selected wavelength. The value of K/S at the wavelength of dye maximum absorbance is directly proportional to the amount of dye present on the dyed fibre before and after GC treatment and ISO3 wash-testing.
Result and Discussion
When aqueous solutions (2% w/w) of typical acid dyes (CI Acid Red 33, CI Acid Black 1, CI Acid Orange 7) were treated with a solution of guanidine hydrochloride (2% w/w) instant precipitation occurred; these precipitates were spotted on Whatman filter paper and are shown in Figure 1.
Figure 1:Selected acid dyes precipitated with guanidine hydrochloride.

It appears that the orange and black dyes are totally insolubilized by the guanidinium cation and the red mainly insolubilized. The use of such insoluble ion-paired chromophores as pigments could be of significance.
The red dye (CI Acid Red 33) was selected for further study. This dye has the structure shown in Scheme 4.
Scheme 4:Structure of CI Acid Red 33.
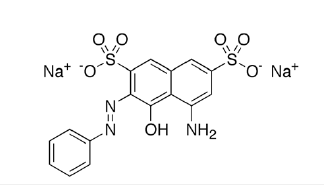
The dye (2%omf) was applied to the nylon fabric from an aqueous bath (liquor ratio 10:1) set at pH 4 (acetic acid), 95 °C for 45 mins. After dyeing the fabric was thoroughly rinsed with running water. The dyed fabric was then split into two portions; one part was after-treated at pH 4.5 at room temperature for 15 minutes with 50gdm-3 guanidinium chloride (GC) solution and the second part after-treated at pH 10 (sodium carbonate) at room temperature for 15 minutes with 50gdm-3 guanidinium chloride (GC) solution). Following these after-treatments both samples were rinsed in water and wash-tested according to the ISO3 test (5gdm-3 sodium carbonate, 5gdm-3 soap at 60C for 15 mins) - this is a very severe wash-test and usually acid dyes on nylon will be significantly desorbed. The residual wash-test liquors are shown in Figure 2 and the actual dyed and washed fabrics are shown in Figure 3. Following the ISO3 wash-testing procedure, colour measurement indicated that 90% of the colour was removed from the normal acid dyeing plus GC at pH 4.5 but remarkably only 2% of the colour could be removed in the ISO3 test when the GC after-treatment was performed at pH 10.5.
Figure 2:Alkaline wash-test liquors from nylon fabric dyed with CI Acid Red 33 -samples. (A) GC after treated at pH 4.5. (B) GC after treated at pH 10.5.
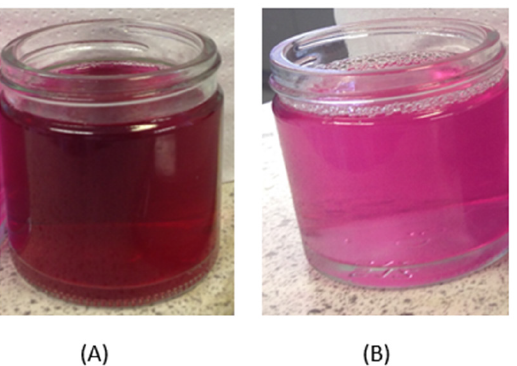
Figure 3:Alkaline washed fabrics dyed with CI Acid Red 33 (LHS - pH 4.5 GC aftertreatment; RHS - pH 10.5 GC aftertreatment).
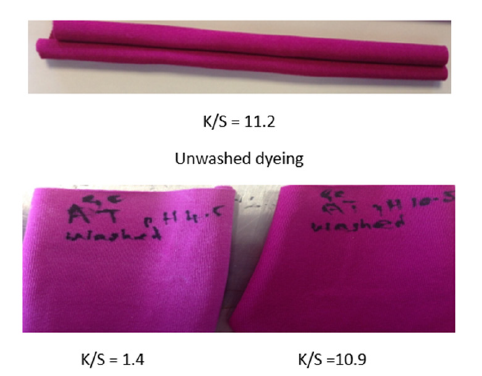
The above results show that only the alkaline aftertreatment with GC gives a notable improvement following alkaline washing fastness testing of the dyed nylon. It is unexpected for acid dyes applied to nylon to resist alkaline washing (ISO3) - only reactive dyes produce dyeings on nylon that approach the above performance. Following the alkaline wash-test, K/S values show that 87.5% of the colour is removed when the GC treatment is carried out at pH 4.5 but only 2.7% of the colour is removed when the GC treatment is carried out at pH 10.5.
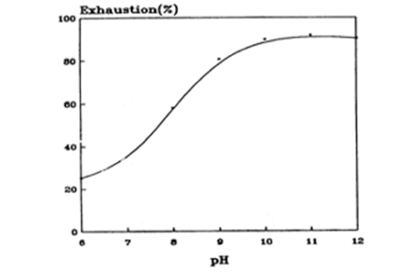
It is worthwhile to discuss how GC alkaline aftertreatment exerts such a large influence. Clearly the guanidinium cation will be highly substantive to nylon when the overall charge on the fibre is negative; at pH values above 9 carboxylic acid residues are fully deprotonated and hence negatively charged; positively charged terminal amino groups are fully deprotonated above pH 9. Previous studies on the uptake of a model cationic reactive dye by nylon [4], reproduced in Figure 4, confirm the excellent substantivity of a cationic dye for the nylon substrate at pH values above 9.
Figure 4:Effect of bath pH on uptake of a model cationic dye by nylon [4].
Conclusion
This study demonstrates that an alkaline guanidine chloride aftertreatment of a disulfonated acid dyeing on nylon 6,6 at room temperature for 15 minutes confers remarkable wash-fastness properties to the dyed substrate. Additionally, rapid precipitation of water insoluble pigment colours occurs when guanidinium chloride in aqueous solution is added to an aqueous solution of a variety of acid dyes. It is necessary to expand the application to a wide variety of polyamide fibres including wool and silk.
Conflict of Interest
The authors declare that there are no conflicts of interest related to this work.
Acknowledgement
This work formed part of a work experience programme for MADC in Perachem PLC laboratories.
References
- Drozdov FV, VM Kotov (2020) Guanidine: A simple molecule with great potential: From catalysts to biocides and molecular glues. INEOS Open 3(6): 200-213.
- Lewis DM (1994) Dyeability of nylon fibres. In: Brody H (Ed.), Synthetic Fibre Materials, Longman, Harlow, United Kingdom, pp. 53-67.
- (2004) Nylon dyeing. In: Lewis DM, Marfell DG (Eds.), Synthetic Fibre Dyeing, pp. 82-121.
- Lewis DM, Sun LJ (2003) Quaternary reactive dyes containing a thioether-ethylsulphone group. Part 2; Application methods for the dyeing of nylon. Coloration Technology 119(6): 327-330.
© 2024 David M Lewis. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






