- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
Decolorization of used Dye Baths Applied for Reactive Dyeing of Cotton
Ivana Vojtová1,2 and Tomáš Weidlich2*
1Pleas s.r.o., Havlíčkův Brod, Czech Republic
2University of Pardubice, Faculty of Chemical Technology, Institute of Environmental and Chemical Engineering, Chemical Technology Group, Pardubice, Czech Republic
*Corresponding author:Tomáš Weidlich, University of Pardubice, Faculty of Chemical Technology, Institute of Environmental and Chemical Engineering, Chemical Technology Group, Pardubice, Czech Republic
Submission: November 22, 2023; Published: December 07, 2023

ISSN 2578-0271 Volume9 Issue3
Abstract
The world’s second largest water consumer is the textile industry. Up to 20 % of global industrial water pollution is produced from textile industry [1]. Water is a common liquid medium in textile production, it is used for removing impurities, dyeing, applying finishing agents or for steam production. Only a small amount of used water is vaporized during individual technological operations. The most of applied water in colorization technology is discharged as wastewater. This wastewater typically contains high COD (Chemical Oxygen Demand) and BOD (Biochemical Oxygen Demand) contamination, high amount of total dissolved salts, exhibits high pH and elevated temperature. Wastewater from textile processes is usually highly colored. Effective wastewater treatment with subsequent recycling reduces the amount of consumed water and quantities of discharged pollutants.
This article deals with wastewater recycling based on removal of dissolved organics after reactive dyeing of cotton fabrics. Typically, it is necessary to use the sodium chloride solution to ensure good dye migration into the fiber. Almost all used sodium chloride remains in the wastewater. This article presents research focused on decolorization of wastewater by precipitation of dissolved anionic dyes applying ionic liquid and final treatment by active carbon. This combined decontamination method produces decolorized brine applicable for subsequent dye bath preparation [2].
Keywords:Textile wastewater; Ionic liquid; Reactive dyeing; Cotton
Introduction
Dyeing wastewater exhibits values of pH, COD and amounts of dissolved inorganic compounds, typically sodium chloride. We developed an innovative technique for simple removal of dissolved colorants from this spent dye baths enabling simple recycling of produced brine. Addition of suitable ionic liquid enables effective precipitation of dissolved anionic dyes including hydrolyzed unfixed reactive dyes by formation of insoluble ion pairs. The remaining organic contamination is subsequently removed by applying active carbon. Subsequent removal of insoluble part produces decolorized aqueous NaCl solution.
Figure 1:Ion exchange reaction of Novacron Black NN reactive dye and commercially available ionic liquid Aliquat 336 produces insoluble ion pair.
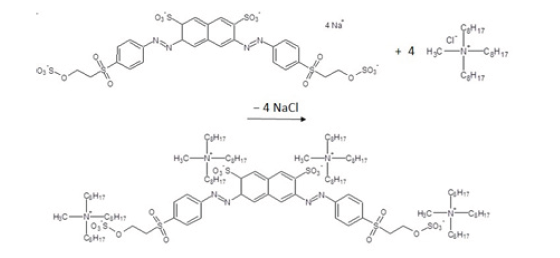
Above mentioned ionic liquids are usually defined as organic salts with a melting point below 100 °C. The physical and chemical properties of these liquids are influenced by both the organic cation and the anion. The advantage of using ionic liquids compared with conventional organic solvents is their almost zero vapour tension, low flammability, ability for ion exchange, good solubility for various organic, inorganic and polymeric compounds and high density. Because of these properties, ionic liquids are sometimes referred to as “green solvents” due to their low environmental impact [3] (Figure 1).
ALIQUAT 336 is a commercially available ionic liquid. It is a quaternary ammonium salt used as a phase transfer catalyst and metal extraction reagent, widely used as a phase transfer catalyst for the catalytic oxidation of cyclohexene to 1,6-hexanedioic acid. It finds application in wastewater treatment by using this agent in the treatment of wastewater containing anionic surfactants. It is a colorless viscous liquid with a melting point of -20 °C and a boiling point of 225 °C [4].
Active carbon (Silcarbon) is a material produced from wood or coconut shells. It has a porous structure and a large specific surface area; it has excellent mechanical strength and a high adsorption capacity. Another advantage is its good availability and relatively low cost [5].
Experimental
The dye bath contains around 3wt.% of the reactive dye, 76g/L of sodium chloride, 10g/L of soda ash and 3,2ml/L of caustic soda (50%). The collected wastewater was strongly colored, strongly alkaline (pH=11.8), the sodium chloride content was in the range of 17g/L. To improve the effectiveness of used ionic liquid A336, the pH value was adjusted to a neutral using Brenntaflor AL flocculant (polyaluminium hydroxichloride). After adjusting the pH value to 6.4, 0.3g of ALIQUAT 336 ionic liquid was added to 100mL of spent dye bath and the mixture was stirred for 30 minutes using a magnetic stirrer. After 30 minutes, the solution was filtered producing practically colourless aqueous filtrate containing sodium chloride (Figure 2) (Chart 1&2).
Figure 2:Photo of a decolorized Novacron Black NN dyeing bath; left- wastewater before cleaning, centre- filtrate after treatment with Aliquat 336, right- filtrate after cleaning with Aliquat 336 and Silcarbon.

Chart 1:VIS spectra of Novacron Black NN dye before and after dyeing.
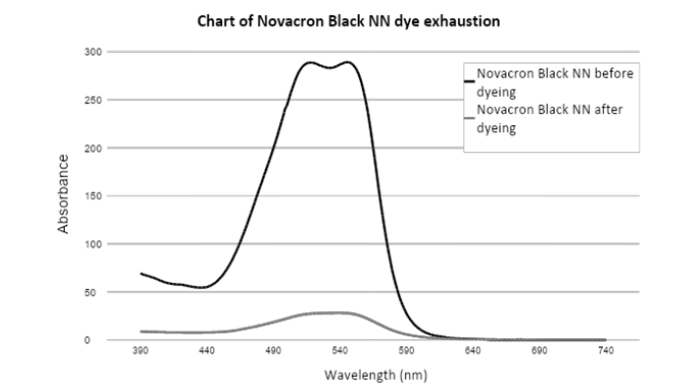
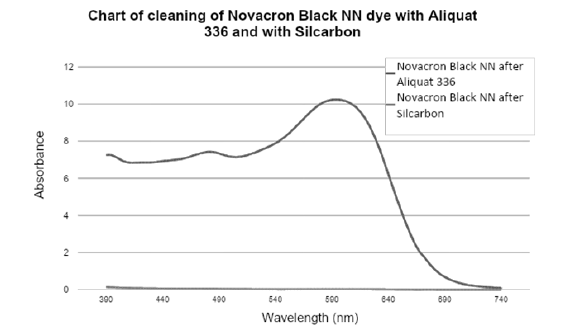
Chart 2:Removal of Novacron Black NN dye from spent dye bath using Aliquat 336 and Silcarbon.
Conclusion
The non-biodegradable pollution of wastewater after textile dyeing is a major issue in how to improve the impact on the environment. The question remains as to how this can be achieved. The performed experiments support effective decolorization of spent dye baths contaminated with reactive dyes using an ionic liquid with subsequent active carbon treatment.
References
- European Environmental Agency.
- Lewis MD (2011) The chemistry of reactive dyes and their application process. Handbook of textile and industrial dyeing 1: 303-364.
- Plechkova N, Seddon RK (2008) Applications of ionic liquids in the chemical industry. Chemical Society Review 1: 123-150.
- Sigmaaldrich, Aliquat 336.
© 2023 Tomáš Weidlich. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






