- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
Intensification of Cellulose Fibres Dyeing with Reactive Dyes Using Quaternary Ammonium Salts
Elokhin IV, Kalugina MS and Mikhailovskaya AP*
Institute of Applied Chemistry and Ecology, Saint Petersburg State University of Industrial Technologies and Design, Saint Petersburg, Russian Federation
*Corresponding author:AP Mikhailovskaya, Institute of Applied Chemistry and Ecology, Saint Petersburg State University of Industrial Technologies and Design, Saint Petersburg, Russian Federation
Submission: February 13, 2023; Published: March 08, 2023

ISSN 2578-0271 Volume 8 Issue2
Abstract
The paper investigates into the dyeing process of cellulose-based fibres with reactive dyes using quaternary ammonium salt as an intensifier. The paper considers the wide usage of сellulose fibres in the textile industry and highlights the trend towards obtaining cellulose fibres from flax, Ramie, hemp. The weak points of reactive dyes application have been analysed and the methods of their elimination have been suggested. Based on the results of their previous studies, the authors claim that quaternary ammonium salts allow reducing the proportion of hydrolysed form of dye. The novelty of this work is in studying the intensifying effect of quaternary ammonium salts in the process of dyeing not only cotton, but also flax, viscose, Ramie, hemp fibres.
The study of reactive dyes sorption by microcrystalline cellulose in the presence of ammonium salts allowed to determine the most effective intensifier for dyeing cellulose fibres. The concentration of the reactive dye on cellulose with benzyldimethylhexadecylammonium chloride increases by 60-70%. This results in the increase of colour strength values of cellulose fibres by 5-6 times. All the examined samples showed high resistance to washing and perspiration. The obtained results are of practical significance and can be used in textile materials processing.
Keywords:Sorption; Benzyldimethylhexadecylammonium chloride; Colour parameters; Fastness properties; Textile industry; Spectrometry
Introduction
Natural fibres of plant origin remain relevant in textile products and composite materials production. It should be mentioned that there is a tendency to obtain cellulose fibre materials from flax, nettle, hemp, bamboo, wheat and rice straw instead of cotton because its cultivation requires the use of large amounts of pesticides and herbicides [1]. For example, hemp does not require the application of any chemicals for its growth. Also, hemp cultivation does not deplete the soil but, on the contrary, fertilizes the soil. The production of fibre from hemp is an economically viable process. Ramie is a plant resistant to parasites and can be grown without the use of pesticides and herbicides as well. Ramie fibre is strong, fine and has a silky sheen [2]. Every plant is based on cellulose that forms strong covalent bonds with active colouring agents with the help of hydroxyl groups. Dyeing of cellulose-based products is done by applying reactive dyes and is characterised by a high resistance of the fibres to physical and chemical influences. However, the application of reactive dyes comes with particular difficulties caused by dye hydrolysis, high concentration of dyes in wastewater, the usage of alkaline agents and electrolytes. Researchers around the world have suggested various solutions to these issues. For example, alkaline and electrolyte application can be avoided with the help of the material pretreatment with dendrimer solution [3] or sodium edetate [4]. The use of carboxylic acid as an intensifier increases the covalent fixation of a reactive dye on cellulose by 12% [5]. Research on the topic primarily deals with the creation of new typ of reactive dyes [6,7], dyeing process and equipment optimization [8,9], chemical modification of fibres with organic compounds [10]. In our opinion, the dyeing technologies for cellulose-based fibrous materials that simultaneously impart antibacterial properties to cellulose are of considerable interest [11]. Previous research showed that the use of quaternary ammonium salts (QAS) allows addressing the deficiency of reactive dyes and reduces the proportion of hydrolysed (deactivated) forms of dye during the dyeing process [12]. Some chemical substances of the examined ammonium salts range not only intensify the process of dyeing cotton fibre with reactive dyes but also allow imparting resistance of natural textile products to the actions of living microorganisms [13]. Researchers all over the world pay much attention to the development of green and eco-friendly dyeing fabric [14].
The results of the experiment in the previous study [15] showed that organic quaternary ammonium salts (chlorides and bromides), having in their structure the long-chain aliphatic radical (dodecyl, hexadecyl), can increase the sorption properties of microcrystalline cellulose by 25-70% relative to reactive dyes. The assumption is made that the increase of the sorption activity of cellulose is connected with the action of a long-chain hydrocarbon radical of nitrogen-containing cation on intermolecular hydrogen bonds of polymer macromolecules. Another research [16] proved that the intensifying effect of cetrimonium bromide and benzalkonium chloride can increase the degree of covalent fixation of reactive dyes on cellulose. Moreover, a similar effect of benzalkonium chloride was observed in the sorption of reactive dyes by another polysaccharide - a starch, which differs from cellulose by its branched structure. The obtained results suggest that cetrimonium bromide and benzalkonium chloride can serve as dye intensifiers for every natural and chemical cellulose-based fibre material.
However, the intensifying effect of quaternary ammonium salts has been explored only for the process of cotton fibres dyeing. The possible application of the mentioned intensifiers for dyeing other cellulose fibres that are widely used in the textile industry has not been investigated yet. The results obtained by the authors suggest that cetrimonium bromide and benzalkonium chloride can serve as dye intensifiers for every natural and chemical cellulose-based fibres: flax, Ramie, hemp, viscose.
The current research aims at estimating the intensifying effect of benzyldimethylhexadecylammonium chloride in the batch dyeing of various cellulose-based fibres with reactive dyes.
To achieve this aim, the following objectives were defined:
A. To choose the ammonium salt as an intensifier for the
batch dyeing of cellulose fibres with reactive dyes.
B. To determine the concentration of different types of active
dyes on fibres using spectrophotometric analysis of residual
dyeing solutions.
C. To evaluate the colouristic and fastness properties of
the obtained dyed samples of cotton, linen, hemp, viscose and
Ramie fibres.
Material and Methods
Fibres
The widely used in the textile industry fibres were examined: cotton, flax, hemp, viscose, Ramie (obtained from the Chinese nettle). All fibres underwent a pre-treatment stage before dyeing, except for hemp and nettle fibres that were not bleached before the dyeing stage and had their natural colour.
Dyes
The cellulose-based fibres were dyed with reactive dyes: Reactive Red 35 (vinyl-sulfone), Reactive Blue 13 (monochlorotriazine).
Dye intensifiers
Quaternary ammonium salts containing a long-chain aliphatic radical in their structure were used as dye intensifiers: hexadecy ltrimethy lammonium bromide, dodecy ltrimethy lammonium bromide, benzyldimethylhexadecylammonium chloride. The purity of the used substances is over 99%, according to the certificates provided by the producer.
Dyeing method
The fibres were dyed using the batch method: 1g of fibre was placed in an aqueous solution containing 1g/l of an intensifier at a treatment temperature. The treatment temperature was determined according to the chemical structure of the dye: 75 °C for Reactive Blue 13 monochlorotriazine dye, 40 °C for Reactive Red 35 vinyl sulfone dye. After 5 minutes, the dye with a 3% concentration by weight of fibre was added to the dyeing bath. The sample was incubated under thermostatic conditions for 30 minutes at the given temperature. Afterwards caustic ash with a concentration of 2g/l was added, and the treatment was continued for 1 hour. The alkaline media is necessary for the formation of a covalent bonding between the hydroxyl group of the cellulose and the reactive group of the dye. The mechanism of the covalent bonds formation between cellulose and reactive dyes has been thoroughly studied in many papers [17,18]. The total volume of the dyeing bath was determined by 20ml per 1g of fibre. After dyeing, the samples were washed thoroughly with cold and hot water.
Determination of the dye concentration on the fibre
The reactive dyes dissolve in the water well. The spectra of their aqueous solutions have highly pronounced maxima in the visible region. The spectra were obtained using Shimadzu UV 2700 spectrophotometer (quartz cuvettes-10mm, measurement temperature 25 °C). The transmission spectrum of the aqueous solution of Reactive Blue 13 reaches a maximum at 570 nm, Reactive Red 35 - at 508nm (Figure 1).
Figure 1:Transmission Spectra of Aqueous Solutions of Active Dyes: 1 - Reactive Blue 13; 2 - Reactive Red35.
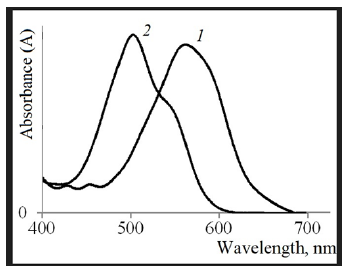
Figure 2:Dependence of the Absorbance of the Solutions on the Dye Concentration: 1 - Reactive Blue 13; 2 - Reactive Red 35.
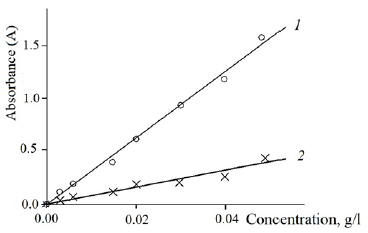
Dependences shown in Figure 2 were plotted by the absorbance of aqueous solutions of dyes of given concentrations from 1mg/l to 0.05g/l at the maximum wavelength of the spectra in the visible region. The obtained dependencies are linear, which allows using them to calculate the concentration of reactive dye in the residual dye solution. The dye concentration values in the residual bath and in the initial dye solution were used to calculate the dye concentration on the fibre. The results of the three tests showed convergence.
Determination of the colouristic and strength properties of the dye
The colour characteristics: reflection coefficient and colour coordinates based on the CIELAB colour space were determined using GretagMacbeth Color i5 spectrophotometer under a standard light source. With the help of the dyed sample reflection coefficient at the maximum of the spectral curve, the colour strength was evaluated as a Gurevich-Kubelka-Munk function. The fastness values were determined according to GOST ISO 105-A01-2013, 9733.4-83, 9733.6-83 standards.
Determination of the mechanical properties
Tensile strength and elongation of white and dyed cotton yarns were determined using MP-3 tensile testing machine according to ISO 6939-88. Ten tests were carried out for each sample.
Result and Discussion
The pilot experiment dealt with a study of monochlortriazine and vinyl sulfone reactive dyes sorption by microcrystalline cellulose in terms of the presence in a dyeing bath organic quaternary ammonium salt having a long-chain aliphatic radical in their structure. Microcrystalline cellulose is characterised by a high degree of crystallinity and pureness. Therefore, in our opinion, ammonium salt having the most significant intensifying effect during dyeing will intensify the dyeing of various cellulose-based fibre materials, both natural and chemical. Table 1 shows the values of Reactive Red 35 and Reactive Blue 13 dyes on microcrystalline cellulose using different quaternary ammonium salts.
Table 1:Dye concentration, mg/g Cellulose.
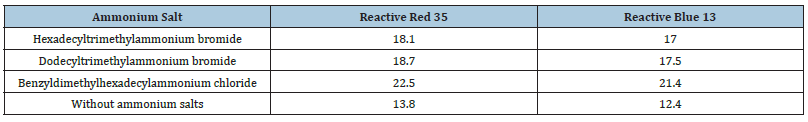
As it is evident from Table 1, the microcrystalline cellulose samples dyed using conventional technology without any intensifier contain 12.4 and 13.8mg/g of the reactive dyes on the polymer. Adding benzyldimethylhexadecylammonium chloride to the dyeing bath increases the dye concentration on the polymer up to 21.4 and 22.5mg/g, which is more than 60%. Taking into consideration the obtained results, benzyldimethylhexadecylammonium chloride, the ammonium salt that produced the most significant intensifying effect, was selected for dyeing cellulose-based fibres.
The concentration of the reactive dye in the solution after the cellulose-based fibres dyeing was evaluated using the residual bath spectra. The dye concentration on the fibrous substrate was further calculated (Table 2).
Table 2:Dye concentration on the dyed samples, mg/g.
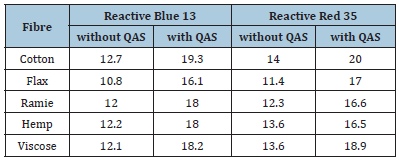
In all cases, the reactive dye sorption is higher when benzyldimethylhexadecylammonium chloride is applied as a dye intensifier. It should be noted that the concentration of the vinyl sulphone reactive dye on the cellulose fibre samples dyed without ammonium salt varied from 10.8 to 14.0mg/g. The addition of benzyldimethylhexadecylammonium chloride to the fibre dyeing bath resulted in the sufficient increase of the reactive dye sorption up to 16.1-20.0mg/g, which is on average 40-50%.
?The obtained colour indices also illustrate the intensifying effect of tetraalkylammonium chloride. Table 3 exhibits the colour coordinates based on the CIELAB colour space and the colour strength values of the fibres dyed with and without applying an intensifier during the batch dyeing process.
Table 3:Colour Indices of the Dyes.
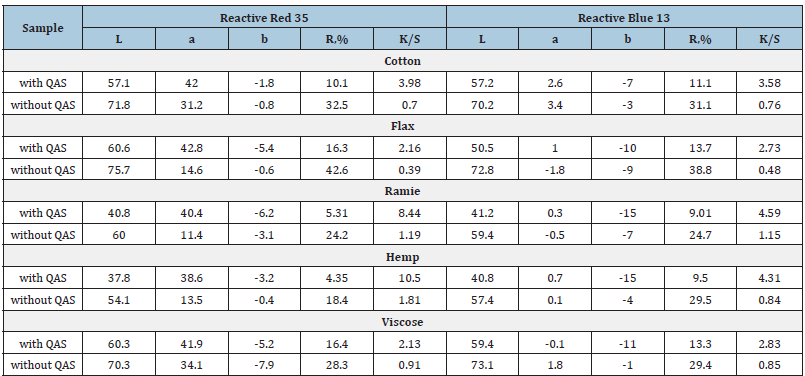
*L – lightness value (from 0 to 100, from black to white); а - position between red and green; b - position between yellow and blue; R - reflection coefficient; K/S - colour strength,F(R)=(1-R)2/2R where R is a fraction.
The findings show that the colour strength of all fibre materials treated with tetraalkylammonium halogenide before adding the colouring agent into the dyeing bath is significantly higher than the samples dyed without adding an intensifier. This is indicated by the values of the lightness coordinate L and the reflection coefficients R. According to K/S values given in Table 3, it is evident that flax and viscose fibre samples have the lowest colour strength. For example, flax fibre dyed with Reactive Red 35 using QAS has a colour strength of 2.16, which is 5 times lower than hemp fibre treated under the same conditions. This can be explained by the hardness of flax fibre and a high degree of polymerization. Also, except cellulose, flax fibre is characterised by the presence of impurities such as lignin and waxes in its content.
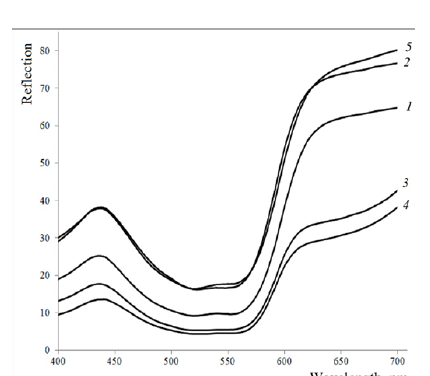
It is worth mentioning that when comparing the samples of flax fibre dyed with and without an intensifier, K/S values increase 6-fold (more than cotton fibre) for both monochlorotriazine and vinyl sulphone dyes. The smallest intensifying effect of benzyldimethylhexadecylammonium chloride application in dyeing with reactive dyes is observed for viscose fibre samples - values of colour strength increase by 2-3 times. The largest colour strength values of nettle and hemp fibres (8.44-10.52) are explained by their natural colour. It should be noted that the reactive dye overlaps the natural colour of nettles and hemp, which is illustrated by the reflection spectra shown in Figure 3.
Figure 3:Reflection Spectra of Fibres Dyed with Reactive Red 35 Dye with Benzyldime-thylhexadecylammonium Chloride: 1 - Cotton fibre, 2 - Flax fibre, 3 - Ramie fibre, 4 - Hemp fibre, 5 - Viscose fibre..
Cellulose-based fibres are often used on their own and in blends with synthetic fibres to produce a wide range of textile materials, including underwear, outerwear, children wear, household textiles, different types of bags, upholstery, etc. Therefore, the colour of such products is often exposed to various detergents and sweat gland secretion. Table 4 shows the colour fastness to perspiration, washing, dry heat, friction.
Table 4:Colour resistance of cotton yarn to physical and chemical influence.
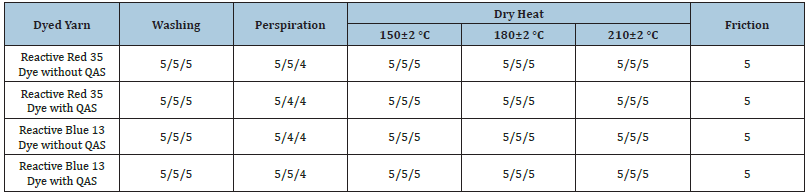
*The highest level of colour resistance is 5.
The fastness values of the colour obtained on cotton yarn are at a high customer value level. Significant attributes of textile materials which are valuable for consumers are tensile strength and elongation [19]. The physical and mechanical properties of cotton yarns dyed using quaternary ammonium salts are preserved at the same level as the undyed material. Tensile strength and elongation values of the samples are: Р=11.26 сН/tex, Δε=11.82% (undyed yarn); Р=11.25сН/tex, Δε=11.84% (yarn dyed with Reactive Blue 13 using benzyldimethylhexadecylammonium chloride); Р=11.06сН/tex, Δε=11.88% (yarn dyed with Reactive Red 35 using benzyldimethylhexadecylammonium chloride).
Conclusion
Based on the results of experiments, it was found:
A. Having a long-chain aliphatic radical, quaternary
ammonium salt increases the sorption of reactive dyes on cellulose
crystallites. Adding benzyldimethylhexadecylammonium
chloride to the dyeing bath can increase the dye content on the
polymer by 40-60%.
B. Benzyldimethylhexadecylammonium chloride increases
the colour strength of cellulose-based fibres. Colour strength
(K/S) values for cellulose fibres dyed without ammonium salt<
range from 0.39 to 1.81 depending on the type of the fibre.
K/S values of cotton, flax, nettle and hemp samples dyed with
benzyldimethylhexadecylammonium chloride are 5-6 times
higher: from 2.16 to 10.52.
C. The colours obtained from dyeing with
benzyldimethylhexadecylammonium chloride in all the
examined cellulose fibres samples have a high washing and
perspiration resistance.
D. In summary, the research showed the possibility to obtain
colours with high colouristic and strength properties on cotton,
flax, hemp, viscose and nettle fibres under conditions of batch
dyeing with reactive dyes and application of the quaternary
ammonium salt.
References
- Moryganov AP, Zakharov AG, Zhivetin VV (2002) Perspective polymer materials for chemical and textile production. Mendeleev Chemistry Journal 46(1): 58-66.
- Krichevsky GE (2021) Green and nature-like technologies-the basis of sustainable development of civilization for future generations. Scientific-Educational Journal NBICS.
- Lewis DM, LTT Vo (2007) Dyeing cotton with reactive dyes under neutral conditions. Coloration Technology 123(5): 306-311.
- Burkinshaw SM, Mignanelli M, Froehling PE, Bide MJ (2000) The use of dendrimers to modify the dyeing behaviour of reactive dyes on cotton. Dyes and pigments 47(3): 259-267.
- Ahmed NSE (2005) The use of sodium edate in the dyeing of cotton with reactive dyes. Dyes and Pigments 65(3): 221-225.
- Khatri A, Peerzada MH, Mohsin M, White M (2015) A review on developments in dyeing cotton fabrics with re-active dyes for reducing effluent pollution. Journal of Cleaner Production 87: 50-57.
- Siddiqua UH, Ali S, Iqbal M, Hussain T (2017) Relationship between structure and dyeing properties of reactive dyes for cotton dyeing. Journal of Molecular Liquids 241: 839-844.
- Yu H, Wang Y, Zhong Y, Mao Z, Tan S (2014) Foam properties and application in dyeing cotton fabrics with reactive dyes. Coloration Technology 130(4): 266-272.
- Shu D, Fang K, Liu X, Cai Y, Zhangl X (2018) Cleaner coloration of cotton fabric with reactive dyes using a pad-batch-steam dyeing process. Journal of Cleaner Production 196: 935-942.
- Tang AYL, Lee CH, Wang YM, Kan CW (2019) Dyeing cotton with reactive dyes: a comparison be-tween conventional water-based and solvent-assisted PEG-based reverse micellar dyeing systems. Cellulose 26(2): 1399-1408.
- Gaffer HE, Elgohary MR, Etman HA, Shaaban S (2017) Antibacterial evaluation of cotton fabrics by using novel sulfonamide reactive dyes. Pigment &Resin Technology 46(3): 210-217.
- Mikhailovskaya AP, Serova NE, Kalugina MS, Kiselev AM (2014) Intensifying effect of quaternary ammonium salts evaluation during cellulose materials dyeing with reactive dyes. Russian Journal of Applied Chemistry 87(1): 114-119.
- Kalugina MS, Mikhailovskaya AP, Zaborski M, Kiselev AM (2016) Imparting biostability to cotton yarn in the process of dyeing with reactive dyes. Design Technology Materials 2: 46-49.
- Jabar JM, Adedayo TE, Odusote YA (2021) Green, eco-friendly and sustainable alternative in dyeing cotton fabric using aqueous extract Mucuna slonaei F dye: effects of metal salts pre-mordanting on color strength and fastness properties. Current Research in Green and Sustainable Chemistry 4: 100151.
- Elokhin IV, Mikhailovskaya AP, AM Kiselev (2020) Effect of ammonium salts on the microcrystalline cellulose sorption properties. Technology of Light Industry 3: 32-36.
- Mikhailovskaya AP, Elokhin IV, Lysova SS (2021) Sorption of reactive dyes by polysaccharides from tetraalkylammonium salts aqueous solution. Chemical Technology 11: 508-512.
- Aspland JR (1992) Reactive dyes and their application. Textile Chemist and Colorist 24(5): 31-36.
- Bobiev OG, Fayzov A, Ravshanov DCH (2022) Interaction of reactive dyes with natural fibers of cellulose origin. The relationship of science with production in the process of accelerated industrialization. pp. 52-55.
- Jabar JM, Alabi KA, Lawal AK (2020) Synthesis, characterization and application of novel 1, 3-bis[(furan-2-l) methylene] thiourea functional dye on wool and cotton fabrics. SN Applied Sciences 2(11): 1-13.
© 2023 Mikhailovskaya AP. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






