- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
Studies in Dyeing of Virgin and Recycled Polyester
Swati V Chavan*, Vinayak Apte, Vaibhav P Kasale, Sajjad A Patel, Avdhut B Pawar and Rohit R Ghadi
D.K.T.E. Society’s Textile and Engineering Institute, Ichalkaranji and Polygenta Technologies Ltd, Nashik Maharashtra, India
*Corresponding author:Swati V Chavan, D.K.T.E. Society’s Textile and Engineering Institute, Ichalkaranji and Polygenta Technologies Ltd, Nashik Maharashtra, India
Submission: November 07, 2022; Published: December 12, 2022

ISSN 2578-0271 Volume 7 Issue4
Abstract
PET is a synthetic fibre, and it is non-biodegradable. It is obtained from petroleum. Along with the widespread application of PET is the inevitable creation of large amounts of PET waste. There are serious economic and environmental problems with the increasing amount of PET waste. The landfill is also a problem as polyester waste goes to landfills and contributes to increasing waste. To create awareness about the waste, recycling is the most efficient option for the treatment of the PET waste. Due to the economic and energy concerns, nowadays the PET recycling is getting done in wide scale.
PET recycling is contributing to saving of raw petrochemical products and energy. Compared to virgin, the products made from recycled plastics result in 55-62% of capital saving. The business sector nowadays is focusing on the sustainability and the public is also concerned about the environment, this is what the main motivation for the PET recycling. The recycled product of PET is available as RPET. This paper deals with studying the dyeing performance of virgin and recycled polyester fabric. The results indicated that recycled polyester exhibited more depth than the virgin PET.
Keywords:Synthetic fibre; Non-biodegradable; Polyester; Textile
Introduction
PET is made from petroleum, a one-time use resource that damages environment during the extraction process. It does not degrade and takes 36-43 years to decompose. Poly- ethylene terephthalate is known as PET in the packaging industry and called as ‘poly- ester’ in the textile industry, is non-dispensable material with lot of applications due to its excellent physical and chemical properties. But also, as consumption is increasing and non-biodegradability, PET waste is now creating serious environmental and eco- nomic threats. So polyester waste management is a serious threat nowadays. But while considering the full life cycle of the PET i.e., from manufacturing to the end use, polyester is not that damaging to the environment. It consumes lower energy during the washing and cleaning and also can be recycled, which is the most efficient way to reduce solid waste. Reprocessing of the by-products again into the useful product is the main objective of the textile recycling. Polyester is the world’s most recyclable polymer. Recycled polyester is used in some products like fibres, films, and foam, sheeting, food, and non-food contact bottles. Main source of textile raw material is the clear plastic water bottles or the used PET which was otherwise going into the landfills. Fleece, a knit ware, home furnishing, t shirts, etc. are the most common type of textile products made from recycled PET. Recycled PET is often used by outdoor clothing companies to make jackets. For the waste treatment of PET, recycling is the most efficient option among the rest. Among the various methods of PET recycling (primary or ‘in-plant’, secondary or mechanical, tertiary or chemical, quaternary involving energy recovery), only chemical recycling conforms to the principles of sustainable development because it leads to the formation of the raw materials from which PET is originally made. From environment point of view the polyester recycling is important and as its use is widespread and as PET bottles, packages and fibres are easily available, it is creating commercial opportunities.
Plan of Work
Material
Plain weave recycled, and virgin polyester fabrics of 75GSM were procured from Polygenta Industries, Nashik.
Chemicals and auxiliaries
Disperse dye Coralene Blue GR from Colourtex Ltd, commercially available carrier and dispersing agent, Acetic acid, Sodium hydroxide, Sodium hydrosulphite all L.R. Grade.
Methodology
The recycled and virgin polyester fabric samples were dyed using Coralene Blue GR dye with 6% shade using 3gpl dispersing agent and 1gpl acetic acid on Data Colour machine. The fabric was introduced in containers of Data colour machine, and the lid was closed. The temperature was allowed to reach to 100 °C at a rate of heating of 4 °C /min. Then the samples were withdrawn one by one at an interval of 5min starting from 5th minute and ending at 60th minute. Same procedure was repeated by maintaining the dyeing temperature of 110 °C, 120 °C and 130 °C upto 60min. Every sample was washed, and reduction clearing was done using 2gpl Sodium hydroxide and 2gpl Sodium hydrosulphite at 60 °C for 20min followed by washing and drying.
Testing and analysis
The dyeing performance of recycled and virgin polyester was analysed by measuring K/S values of the dyed samples before and after reduction clearing on Premier Coloureye 3000 machine. The tensile strength of fabric was measured by ASTM D5035 [1] using Instron 5565 equipment. DSC and X-ray Diffraction analysis was done in Shivaji University laboratory.
Figure 4:The discharge curves for various current densities. (0) The experimental curve 2mA/cm2 and the calculated curves (I) -2mA/cm2 , (II) -4mA/cm2, (III) -6mA/cm2, (IV) -8mA/cm2, (V) -10mA/cm2.
Result and Discussion
Basic objective of this research was to evaluate or study the structural aspects of virgin and recycled polyester and dyeing tool has been selected for the same as dyeing occurs in amorphous region. Polyester is thermoplastic polymeric material, and it is viscoelastic material, viscoelasticity is achieved because of crystalline as well as amorphous region and Crystal size, shape plays very important role in mechanical and physical properties of fibre [2-6]. Dyeing also gets affected largely because of amorphous and crystalline region.
Dyeing behavior of virgin and recycled polyester
Results from Table 1 indicate the K/S values of virgin and recycled polyester samples dyed using Coralene Blue GR dye at 6% shade taken out at an interval of 5min each at temperatures of 100 °C, 110 °C, 120 °C and 130 °C before and after reduction clearing process.
Results from Table 1 and Graph 1 indicate that with the increasing temperature, the rate of dyeing also increases. Rate of exhaustion is highest at 110 °C but dyeing at 130 °C is preferred to avoid migration of dye. Dye starts migrating at 80 °C but only at surface, at 90 °C the pores get opened, dye may penetrate, so to carry out dyeing minimum 100 °C temperature is required. So, the dyeing is started at 100 °C and dyeing behavior is observed at 10 °C increased level till 130 °C.
Table 1: Dyeing behavior of virgin and recycled polyester.

Graph 1:Effect of time and temperature on dye uptake of virgin and recycled polyester.
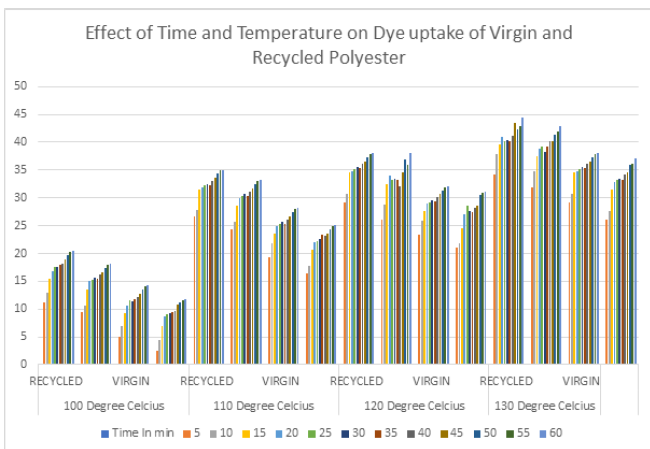
Exhaustion rate of dye at 100 °C for both virgin and recycled polyester fabrics was observed and the colour yield was measured in terms of K/S values before and after reduction. Difference in depth between the reduction cleared sample and non-reduction cleared sample is only more at 100 °C which is further reduced as temperature increases from 100 °C to 130 °C. At 100 °C the dye gets exhausted but more at superficial position, but as temperature increases more and more superficial dye gets penetrated inside the fibre structure and hence even after reduction clearing there is no much difference in colour yield [7]. Dyeing colour yield of recycled polyester is comparatively more than that of virgin polyester, which may be attributed to the fact that there may be presence of faulty crystals in molecular chain length, chain break etc while manufacturing recycled polyester This implies that long crystals are not formed. Hence difference in dyeing behavior of recycled and virgin polyester is observed. From the results it can be stated that dyeing of recycled polyester is easier than that of virgin polyester and dye fixation is also more in case of recycled polyester [8-10].
Load-Elongation curve of virgin and recycled polyester
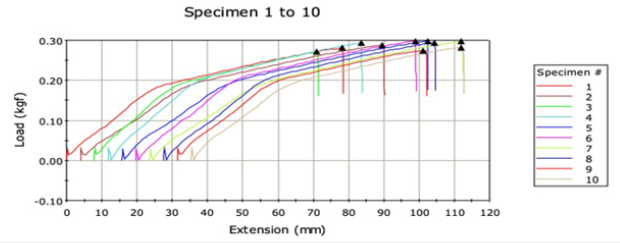
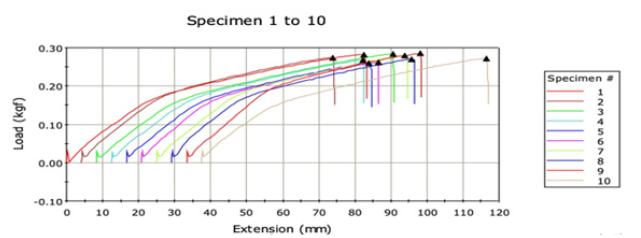
Table 2 and Graph 2 indicate the data of tensile properties of virgin and recycled polyester fabrics. More is the heat setting of fibre, less elongation and more load bearing capacity is exhibited. Virgin polyester bears more load and shows less elongation as indicated in Graph 2 which indicates that there is more percent of the crystalline region [11]. Recycled polyester elongates more at less load because of more amorphous region as it can be observed in Graph 3, they don’t have stronger crystals and have smaller crystals. So as more amorphous region in the recycled polyester, it shows more depth in shade, which is also supported by loadelongation curve [12].
Table 2: Tensile strength data of Recycled and Virgin polyester.

Graph 2:Load elongation curve of Recycled polyester.
Graph 3:Load elongation curve of Virgin polyester.
DSC of virgin and recycled polyester
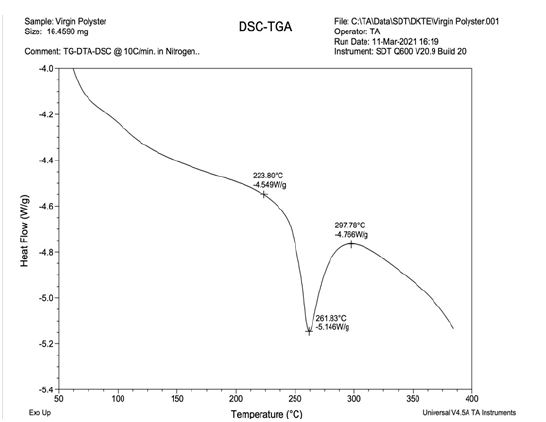
As indicated in Figure 1&2, X axis denotes temperature and on Y axis there is corresponding supply of heat flow. Heat is continuously supplied but rise in temperature is not constant. Different polymers show different rise in temperature. Now in case of Tg of polymer, polymer molecule absorbs heat, therefore heat is absorbed and rise in temperature is reduced, so small peak is generated at Tg. If heating is continued the rise in temperature decreases drastically at the time of softening point and melting point. In case of melting point polymer molecules are attached firmly to each other in crystalline region and once crystals start melting, rise in heat is decreased drastically and peak is observed. In case of virgin polyester, from Figure 2 it can be observed that the crystals are perfect and clear, size is larger, amount of heat absorbed is more and as all crystals have similar size hence width of peak is reduced. So better the crystal, sharp peak is there, depth of peak is higher and width lower [13]. On the opposite side in case of recycled polyester there is degradation of polymeric chain length resulting in development of imperfect smaller crystals. Smaller unoriented imperfect crystals because of wide range of size, bigger crystals as well as smaller crystals, the peak observed is broad. Because initially smaller crystals start melting, then bigger crystals and larger crystals at the end which indicates lower depth of peak and higher width as shown in Figure 1. Higher width indicates presence of wide range of crystals size including smaller to larger. In case of Virgin polyester peak is sharp and depth is high because all crystals are in narrow range of distribution of size. And in case of recycled polyester, wide range of distribution of size is there so peak is wider and depth is less. Comparing these to dyeing, we can observe that, dyeing depth in recycled polyester is more because it has smaller crystals, excess space is available between two crystals and depth. Thus, it can be stated that dye uptake by recycled polyester is more than that of virgin polyester and dye fixation is also more in case of recycled polyester [14].
Figure 1:DSC-TGA curve of Recycled Polyester.
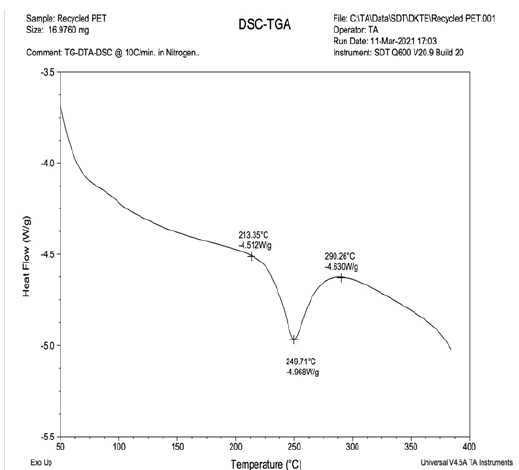
Figure 2:DSC-TGA curve of Virgin Polyester.
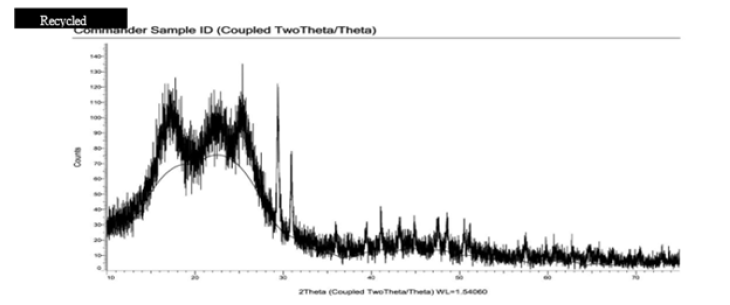
XRD of recycled and virgin polyester
To support DSC, XRD is also carried out for virgin and recycled polyester. In case of virgin polyester, smaller crystals are very less in number, and noise generated in amorphous region is small. So as shown in Figure 3 initial small peaks indicate unoriented, very smaller crystals, mesomorphous region. At 2θ on x-axis, 2 sharp peaks are observed which show there is perfect crystallinity and again followed by smaller peaks which show amorphous and mesomorphous region [15,16]. Therefore 2 sharp peaks indicate perfect crystallinity. As we can observe in Figure 4 at the similar location in case of recycled polyester, 2 peaks are observed, but their height is minimum compared to other peaks of smaller crystals. This indicates wide distribution of smaller crystals. There is no presence of sharp peak, hence similar behavior of smaller crystals is observed, the extent of amorphous region is more hence is the dye uptake. So, from XRD also it is clear that there is no sharp crystalline region which indicates more percent of amorphous region, so dye absorption is also more [17-19].
Figure 3:XRD of Virgin Polyester.
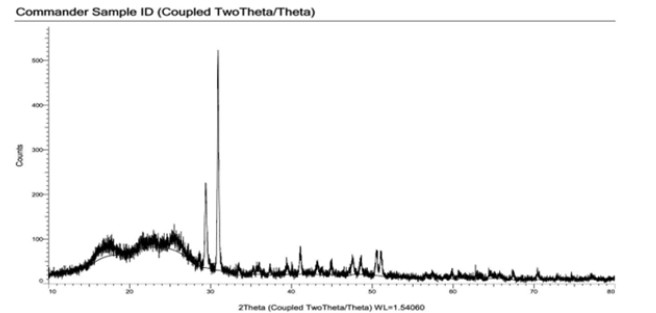
Figure 4:XRD of recycled Polyester.

Conclusion
• From the dyeing behavior it can be concluded that, for same
temperature and time, the recycled polyester exhibits more
depth of colour than that of virgin polyester.
• The recycled polyester shows more elongation at lower load
than the virgin polyester which bears more load and elongates
less at same load as recycled polyester doesn’t have stronger
crystals and bigger crystals as compared to virgin polyester.
• DSC graphs established that virgin polyester has narrow
distribution of crystal sizes and most of the crystals are larger in
size. Whereas recycled polyester has wide distribution of crystal
sizes varies from smaller to larger.
• From the X-Ray Diffraction graphs it is concluded that virgin
polyester has more crystalline region than recycled polyester.
i.e., the amorphous region in recycled polyester in more than the
virgin one hence shows more depth of colour as compared to
virgin polyester at same temperature and time.
References
- Sharma ND, Shubhada H (1995) Management of PET waste. Asian Textile Journal 4(6): 39-52.
- Adanur S (1995) Textile waste management. Wellington Sears Handbook of Industrial Textiles (1st edn), p. 713.
- Patil PD, Deshpande RH, Indi YM, Hattimare R (2018) A green approach: Comparative study of virgin and recycled polyester for textile application. JETIR 5(12): 719-723.
- Bassett JG (1992) Reuse of polymer and fibre waste. International Fiber Journal 7(5): 28.
- Venkatachalam S, Nayak SG, Labde JV, Gharal PR, Rao K, et al. (2012) Degradation and recyclability of polyethylene terephthalate, Polyester. Hosam Eldin (Ed.), p. 76.
- Fried JR (2003) Polymer science and technology. (2nd edn), pp. 274-275.
- Bartolome L, Imran M, Cho BG, Al Masry WA, Kim DH (2012) Recent development in chemical recycling of polyester. Material Recycling-Trends and Perspective pp. 67-68.
- Odion G (2004) Principles of polymerization. John Wiley Inter science, (4th edn), pp. 92-97.
- Reese G (2004) Polyester fibers, encyclopedia of polymer science and technology. (3rd edn), Wiley, New York, USA, 3: 652-678.
- East AJ (2004) Synthetic fibers: Nylon, polyesters, acrylic, polyolefin. JE McIntyre Edition, The Textile Institute, Wood Head Publishing Limited, Cambridge, UK, pp. 95-167.
- Klaus D (1993) Comperl, making the polymer. Polyester: Tomorrow’s Ideas and profit: 50 years of achievement, The Textile Institute, Manchester, UK, pp. 56-59.
- Sabourin D (1993) Recycling: Wellman pioneers the cause of earth’s survival, Polyester: Tomorrow’s Ideas and profit: 50 years of achievement, The Textile Institute, Manchester, UK, pp. 105-107.
- Jones K (1993) PET bottles, Polyester: Tomorrow’s Ideas and profit: 50 years of achievement. The Textile Institute, Manchester, UK, pp. 266-269.
- Domeshek KA (1993) Polyester recycling: an environmental success story, Polyester: Tomorrow’s ideas and profit: 50 years of achievement. The Textile Institute, Manchester, UK, pp. 288-290.
- Shen L, Worrell E, Patel MK (2010) Open loop recycling: A LCA case study of PET bottle to fibre recycling. Resources, Conservation and Recycling 55(1): 40-41.
- Spaseska D, Civkaroska M (2010) Alkaline hydrolysis of poly (ethylene terephthalate) recycled from thepostconsumer soft-drink bottles. Journal of the University of Chemical Technology and Metallurgy 45(4): 371-384.
- Fann DM, Huang SK, Lee JY (1996) Kinetics and thermal crystallinity of recycled PET I. dynamic cooling crystallization studies on blends recycled with engineering PET. Applied Polymer Science 61(8): 1375-1385.
- Fann DM, Huang SK, Lee JY (1996) Kinetics and thermal crystallinity of recycled PET, II. Topographic study on thermal crystallinity of the injection molded recycled PET. Applied Polymer Science 61(2): 261-271.
- Oromiehie A, Mamizadeh A (2004) Recycling PET beverage bottles and improving properties. Polymer International 53(6): 728-732.
© 2022 Swati V Chavan. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






