- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
The Effect of Molecular Weight of Chitosan Polymer on the Production of Nanofibers by Electrospinning
Niloofar Rafizadeh Zaeem1* and Kianoosh Karimi2
1Department of Polymer Engineering, South Tehran Branch, Islamic Azad University, Tehran, Iran
2Department of Textile Engineering, Science and Research Branch, Islamic Azad University, Islamic Azad University, Tehran, Iran
*Corresponding author:Niloofar Rafizadeh Zaeem, Department of Polymer Engineering, South Tehran Branch, Islamic Azad University, Tehran, Iran
Submission: September 23, 2022; Published: October 27, 2022

ISSN 2578-0271 Volume 7 Issue3
Abstract
Chitosan is a natural origin polymer and is one of the derivatives of chitin; Chitosan is the most abundant polysaccharide in the world after cellulose. Chitosan has outstanding properties such as biodegradability, abundance, antibacterial and antifungal effects and reactivity, which leads to many applications such as: hygiene-health, agriculture, food production, cosmetics, water purification and waste treatment. Bigger contact surface of a polymer causes more interaction, therefore a layer containing nanofibers will provide much better contact surface. In this study, average molecular weight and high molecular weight of chitosan nanofibers were produced. In both cases, a mixture of TFA/DCM solvents with the ratio of (70/30) respectively was employed. viscosity of mentioned solvent was investigated, then the solutions of chitosan and solvent mixture were electrospun. Morphology of obtaining fibers was studied by SEM; also, the physical structure was investigated by XRD. After that, recovered moisture of nanofibers was investigated. Finally heavy metal (Cu (II)) adsorption behavior of nanofibers was compared using an atomic adsorption spectrometer. The optimal spinning solution contained 7wt% chitosan. Average diameter of high molecular weight chitosan fibers was 128nm. According to the results, high molecular weight chitosan nanofibers absorbed more Cu (II) ions compering to average molecular weight chitosan nanofibers.
Keywords:Antibacterial; Agriculture; Food production; Cosmetics; Spinning; Nanofibers
Introduction
The properties of chitosan are highly influenced by its production method because it is the method that determines the deacetylation extend [1]. The deacetylation extends controls the amount of free amine groups in the polymer chain which suggests the positive charge of chitosan. The presence of amine groups next to hydroxyl groups make the polysaccharide chitosan highly reactive. Positive charge of chitosan causes an electrostatic interaction with negative molecular charge [2]. The production method determines the number of active groups caused by deacetylation. The number of these groups affects the crystallinity; and the crystallinity is directly related to the solubility of chitosan in acidic solutions. This in fact is the importance of the method of production of chitosan [3].
Due to the high biological properties of chitosan, this material has become an effective substance in health-related matters. These properties include biodegradability, non-toxicity, antifungal effects, accelerated wound healing and simulation of disease-resistant systems [4]. Biological and chemical properties of chitosan can control cholesterol, fat, protein and metallic ions. Also mentioned properties reason for tumor cells destruction. This has led chitosan to be used as a chelating agent [5].
Active groups and natural chelating property of chitosan make it an efficient material in the process of metal ions separating from effluents; metal ions such as Cu, Pb, Hg and U. Hg (II) is a toxic element and has destructive effects on central nervous system, liver and kidneys. Hg (II) pollution occurs as a result of wasted materials such as: electrical components, paints, plastics, oil refiners, batteries and etc. In order to eliminate Hg (II) pollution out of nature, methods such as sedimentation, ion exchange and adsorption are used. The adsorption method is the most economical [6]. Pb (II) is a toxic element and causes nausea, muscle problems, damage to the nervous system, destruction of lung tissues, destruction of eye tissues, skin allergies, lung failure and liver damage [7]. Chitosan could be used in the sedimentation of food particles containing protein, removal of pigments and separation of all negative charged particles out of effluents.
Conventional methods for removing metal ions from effluents include reverse osmosis membrane, ion exchange, evaporation and chemical deposition. For removing metal ions from dilute solutions, most of these methods are ineffective or costly [8]. Utilizing of sorbents is one of the most common methods for removing heavy metal ions from dilute solutions, which is based on electrostatic interaction and chelation. The advantages of this method are low cost, easy recovery of metals from the adsorbent and the possibility of reusing the adsorbent [9].
Most important problems of electrospinning are its high viscosity and low electrical conductivity [10]. In this research, different methods such as: using solvents with different solubility and mixture of solvents, optimization of spinning solution concentration, viscosity reduction of spinning solution by hydrolysis of polymer chains and using compounds which enhance electrical conductivity were performed not only to overcome mentioned problems but also to prepare nanofibers from biodegradable chitosan polymer. In the presented work, we have tried to obtain the thinnest fibers possible with the highest uniformity. In order to obtain optimal nanofibers, fiber morphology was investigated with SEM. Next, the ability of prepared nanofibers to remove heavy metals (such as copper (II)) in aqueous solutions was evaluated.
Materials and Equipments
Materials
High molecular weight chitosan polymer (800-2000cps viscosity) and average molecular weight chitosan polymer (200- 800cps viscosity), pure (99<) tetra fluoro acetic acid (TFA), pure (99.5<) chloromethane, pure (99.8<) glycolic acetic acid (ACOH), 37-38% hydrochloric acid (HCl), pure (99<) dimethyl sulfoxide (DMSO), pure (98<) copper chloride (CuCl2), pure (99<) potassium carbonate (K2CO3) (all Merck brand made in Germany).
Equipments
Scanning electron microscope (SEM) (LEO 440i model made in England), Cannon Fenske 400 viscometer, atomic absorption spectrometer (Spectra AA200 made in Australia), XRD diffractometer (Seifert 3003pts made in Germany), uniaxial electrospinning device (Fanavaran Nanoscale Company, made in Iran) and conductivity meter (Hanna HI2030 made in USA).
Preparing of Spinning Solution
ACOH90%
A. Chitosan dissolution in ACOH without hydrolysis: 2, 1.25,
0.75, 0.5, 0.25 and 0.125wt% of high molecular weight chitosan
polymer powder was dissolved in 90% glycolic acetic acid. Magnetic
stirrer was used.
B. Chitosan dissolution in ACOH with hydrolysis: in order
to reduce the molecular weight, high molecular weight chitosan
polymer powder was dissolved in soda solution (CS/NaOH 50-
50wt% ratio); then it was heated at 95 °C temperature for 24, 48
and 72 hours. After that, 3, 2.75, 2.25 and 2wt% of the resulting
polymer powder was dissolved in 90% glycolic acetic acid.
0.2M HCl
3.5, 3, 2.5, 2, 1.5, 1, 0.83, 0.6 and 0.5wt% of high molecular weight chitosan polymer powder was dissolved in 0.2M HCl solution.
0.6M HCl
3.5, 2.5 and 2wt% of high molecular weight chitosan polymer powder was dissolved in 0.6M HCl solution.
0.6M HCl with 3% NaCl
3.5, 2.5 and 2wt% of high molecular weight chitosan polymer powder was dissolved in 0.6M HCl with 3% NaCl solution.
TFA
a) TFA without solvent: 9, 7.5 and 3wt% of high molecular
weight chitosan polymer powder was dissolved in pure TFA.
Magnetic stirrer was used.
b) TFA with solvent: Dichloromethane solvent (DCM) was
used. 9, 7.5 and 3wt% of high molecular weight chitosan polymer
powder was dissolved in TFA/DCM (70/30) solution. Magnetic
stirrer was used.
Viscosity and molecular weight measurement
Both high and average molecular weight chitosan spinning
solutions (with TFA/DCM (70.30) solvents) were prepared. The
viscosity of the solutions was measured after 72, 60, 36 and 12
hours prior to preparation process. Volumetric viscometer was
calibrated first. For calibration, super liquid paraffin (d=0.85g/
cm3, Kin viscosity=16mm2/s) and viscous paraffin (d=0.860g/cm3,
Kin viscosity=42.5mm2/s) were used. This is resulted in constant
values A and B in (Equation 1).
η´ = A(pt) − B(ρt ) (Equation 1)
ή: Viscosity(mm2/s)
ρ: Density(g/cm3)
t: Time (min)
Mark Hoying equation was used to measure the mean molecular weight of chitosan. Resulted viscosity values from Equation 1 were inserted in Mark Hoying equation, which resulted the average molecular
weight. A=0.71 and K=8.93X10-2 cm3/g are constants related to chitosan [11].
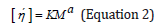
[ή]: Viscosity(mm2/s) M: Mean molecular weight
Preparation of Nanofibers
The air bubbles were elicited from the polymer solution using the ultrasonic device and it became homogeneous; then the solution was inserted into the glass syringes. The positive electrode of the device was connected to the metal of the syringe (nozzle) and the negative electrode was connected to the collector plate. According to past experiences, the optimal conditions for electrospinning were performed, which are: 30KV (voltage), 20μL (feeding rate) and 15cm space (distance between the needle tip and the collector plate).
Fiber’s morphology investigation
Scanning electron microscopy (SEM) was used to investigate the morphology and structure of the samples. The samples were covered with a thin layer of gold.
Neutralization with potassium carbonate solution
A solution of 1mol/L potassium carbonate (K2CO3) was prepared. Chitosan nanofibers, high molecular weight polymer powder and average molecular weight polymer powder were immersed in mentioned solution (separately) for three hours at 20 °C. Then these samples were washed with distilled water to reach pH=7. Finally, the samples were dried in a vacuum oven at 60 °C for 24 hours [12].
Thickness measurement of crystals
High molecular weight chitosan nanofibers, average molecular
weight chitosan nanofibers (both obtained from 7wt% chitosan
solution), high molecular weight chitosan polymer powder
and average molecular weight chitosan polymer powder were
investigated by XRD diffraction under the following conditions:
Diffract: Transmission
Detector: Scintillation
Monichrom: Curved Germanium (III)
Radiation: 40kv, 30mA
Scan Mode: Transmition
2 Theta (begin, end, step) =0.00, 35.00, 0.005 30.00sec/step
Imax=472
Scherer formula (Equation 3) was employed to calculate the thickness of crystals [13].

Measurement of recovered moisture method
Recovered moisture of high molecular weight chitosan nanofibers, average molecular weight chitosan nanofibers (both obtained from 7wt% chitosan solution treated with potassium carbonate), high molecular weight chitosan polymer powder and average molecular weight chitosan polymer powder (treated with potassium carbonate) were measured. To perform this, the above items were dried at 80 °C in an oven for 6 hours; then the dry weights were measured. By inserting these weights in Equation 4, recovered moisture at the desired time was calculated [14].
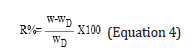
R%: recovered moisture percentage W: weight of a sample at a specific time WD: weight of a dried sampl
Measurement of copper (ii) ion absorption
High molecular weight chitosan nanofibers, average molecular weight chitosan nanofibers, high molecular weight chitosan polymer powder and average molecular weight chitosan polymer powder were immersed in a solution containing 112ppm of copper (II) ion for 1440, 960, 240, 120, 60, 30, 15 and 5 minutes. After immersion, chitosan was separated from the solution by filter paper and the concentration of residual copper (II) ion was measured by atomic absorption spectrometer. The amount of adsorbed ions (in milligrams) could be calculated using Equation 5 [15].
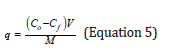
q: adsorbed amount (mg/g) C0: initial density of metallic ion (mg/L) Cf: final density of metallic ion (mg/L) V: solution volume (L) M: amount of adsorbent used (g)
Fiber’s morphology investigation
Scanning electron microscopy (SEM) was used to investigate the morphology and structure of the samples. The samples were covered with a thin layer of gold.
Result and Discussion
Results of viscosity and molecular weight
The viscosity and molecular weight of some of the spinning solutions were compared. The results are presented in Table 1.
Table 1:Viscosity and molecular weight of spinning solutions.

Result of nanofiber preparation methods Spinning solution containing 90% ACOH
Solution containing dissolved chitosan without hydrolysis in ACOH: All spinning solutions of this section (0.125, 0.25, 0.75, 1.25 and 2wt%) were not capable of spinning and they only caused dripping on the collector.
Solution containing dissolved chitosan with hydrolysis in ACOH:Spinning was not possible because chitosan (treated with 50% soda) didn’t dissolve.
Spinning solution containing HCl:HCl is a good solvent for
chitosan. Reasons are:
a) Electrical conductivity increment
b) Better dissolution of mineral salts such as NaCl in HCl
c) Resulting a spinning solution with suitable viscosity with
less amount of polymer powder and solvent.
Spinning solution prepared with 0.2M HCl:All spinning solutions in this section (0.5, 0.6, 0.83, 1, 1.5, 2, 2.5, 3 and 3.5wt%) were not capable of spinning and they only caused dripping on the collector.
Spinning solution prepared with 0.6M HCl:All spinning solutions in this section (2, 2.5 and 3wt%) were not capable of spinning. 2 and 3wt% solutions were suitable for spinning, but the spinning process wasn’t favorable. Although, spinning was better compering 90% ACOH.
Spinning solution prepared with 0.6M HCl and 3% NaCl:All spinning solutions in this section (2, 2.5 and 3wt%) were not capable of spinning. Although, it was better than 0.6M HCl alone.
Spinning solution containing TFA
Spinning solution containing TFA alone:Spinning solutions
containing 9, 7, 5 and 3wt% of high and average molecular weight
chitosan were electro spun. The storage time of spinning solutions
to achieve the best viscosity was 36 hours. SEM images and mean
diameter of resulted fibers are shown in Figure 1 and Table 2. The
spinning solution containing TFA, unlike ACOH, could be spun. The
advantages of using TFA instead of ACOH are:
A. Electrical conductivity increment
B. Dielectric constant increment
C. Surface tension reduction
Figure 1:SEM images of chitosan fibers with different concentrations (both average and high molecular weight).
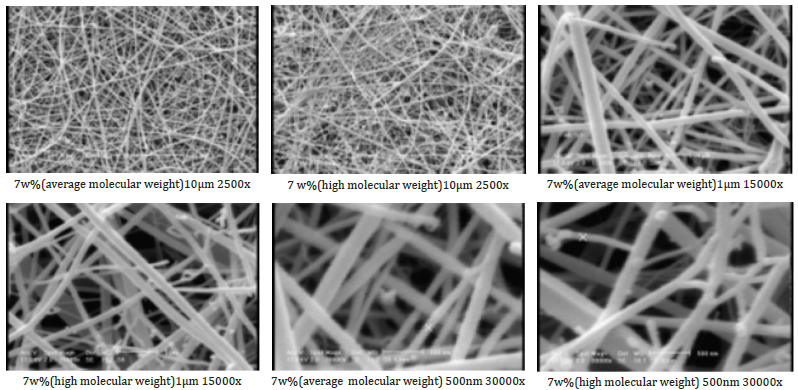
Table 2:Mean diameter and standard deviation of fibers.

As illustrated in the figures, high molecular weight chitosan fibers have a smaller diameter than average molecular weight fibers. In terms of diameter uniformity and presence of beads, average molecular weight chitosan fibers have better quality.
Spinning solution containing TFA with DCM solvent:Spinning solutions containing 9, 7, 5 and 3wt% of high and average molecular weight chitosan were electro spun. The storage time of spinning solutions to achieve the best viscosity was 36 hours. SEM images and mean diameter of resulted fibers are shown in Figure 2 and Table 3.
Figure 2:SEM images of chitosan fibers with different concentrations (both average and high molecular weight).

Table 3:Mean diameter and standard deviation of fibers.

According to the figures, increasing wt% of chitosan from 3wt%
to 7wt% in both average and high molecular weight polymers
has reduced the diameter and bead in the resulted fibers. Also
increasing %w of chitosan in the spinning solution has increased
viscosity and entanglement of molecular chains. Increasing of
entanglement, reduces the diameter and bead in three ways:
a) Increasing the applied tension to the spinning jet: When
the polymer entanglement increases, the electric charges on the
spinning jet reason a proper tension to the polymer solution.
b) Increasing the stability of the spinning jet: As the speed
of jet (from needle toward collector) increases, it results in tension.
During tension, entanglement of molecular chains prevents the jet
from collapsing due to rotation in an electric field, which causes the
spinning jet to be more stable.
Reduction of solvent between molecular chains:As the wt% of the polymer increases (which causes to an increment of viscosity and entanglement of molecular chains), solvent molecules in solution decreases and this reduces the surface tension of polymer solution. Reduction of surface tension causes the formation of droplets and beads during the spinning process. Fibers obtained from 5wt% solution of average molecular weight chitosan have an excellent diameter uniformity, but instead are not continuous. This discontinuity is due to low wt% of chitosan and the collapse of the jet during the spinning process.
Samples obtained from 3wt% solution of high molecular weight chitosan are small droplets due to its very low wt% and high surface tension of the solution. Fibers obtained from 9wt% solution of high molecular weight chitosan are non-uniform and ribbon-shaped due to the very high wt% of chitosan. Based on the figures, at constant wt%, increase in molecular weight of chitosan reduces the diameter and bead in resulted fibers and increases stability of the spinning process. The polymer molecular weight indicates the length of polymer chains; also affects the viscosity of the solution. Increasing the polymer molecular weight increases the entanglement of polymer chains and viscosity, which causes reduction of diameter and bead in the fibers. Increasing the polymer molecular weight also increases stability of the spinning process.
Based on the figures, increasing DCM solvent of both average and high molecular weight chitosan spinning solution increases the fiber diameter; on the other hand, it reduces beads and improves diameter uniformity of resulted fiber; also, it increases the stability of the spinning process. Increasing the DCM increases the surface tension and decreases the electrical conductivity; also, it increases the dielectric constant. Increasing the dielectric constant improves the uniformity and morphology of the fibers in two ways:
Increasing the solubility of solvent
Since increasing the dielectric constant reduces the gravitational forces between the solute ions (according to Cologne law, Equation 6), as a result, it increases the solubility of solute. Cologne’s law describes the electrostatic reaction between charge
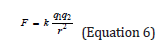
F: force between ions (N) q1×q2: electric charges (c2) r2: square of distance between to charges (m2) K: cologne’s constant ((Nm2)/c2)
Increasing the solubility of solvent is effective in this matter in two ways:
a) A stronger solvent dissolves higher wt% of the polymer while maintaining its fluidity, resulting a more stable solution. b) Increasing the solubility of the solvent improves the encapsulation of polymer chains caused by solvent molecules and as a result reduces the ferromagnetic structure in the chains.
Increasing load storage of spinning jet
In a constant electric field, dielectric constant indicates the storage capacity of a material when exposed to that electric field. Increasing storage capacity of spinning jet causes electric field energy to affect deeper sites of the jet.
Investigation of tests performed after nanofiber layer formation
In this section, we review the tests performed on the prepared nanofiber layer. The best chitosan nanofibers made were resulted from 7wt% spinning solution containing TFA/DCM solvent (both average and high molecular weight chitosan). Therefore, in this section, only the mentioned nanofiber layers are examined.
Neutralization with potassium carbonate solution
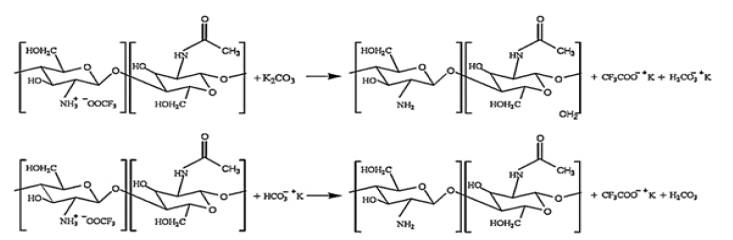
Potassium carbonate (K2Co3) with trifluoroacetate (CF3Coo−) were used to neutralize the salt (-NH+ CF Coo−) originated from the ammonium (-NH3+) of fibers. The formation of the above salt causes dissolution of chitosan in trifluoro acetic acid (TFA). Ammonium ions (-NH3+) on chitosan nanofibers repel positively charged metal ions, so potassium carbonate is used to neutralize the salt. The neutralization process happens in this order: the potassium carbonate reacts with the salt; this causes the production of potassium trifluorostat (CF3Coo− K+) salt; then chitosan nanofibers are neutralized by amine groups (-NH2). The presence of amino groups on chitosan nanofibers increases the adsorption capacity of heavy metal ions. The above reactions are shown schematically in Figure 3 [12].
Figure 3:Neutralization of chitosan nanofibers [12]..
XRD result
Nanofibers obtained from 7wt% chitosan solution and both high and average molecular weight chitosan polymer powder were examined by XRD diffractometer, and the results are shown in Table 4 and Figure 4.
Table 4:XRD results.
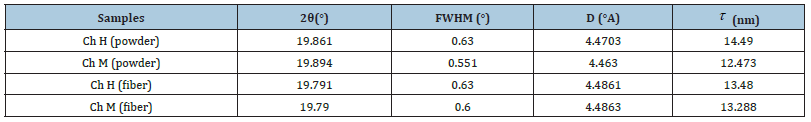
Figure 4:XRD diagram of: a) High molecular weight chitosan powder. b) Average molecular weight chitosan powder. c) High molecular weight chitosan nanofibers. d) Average molecular weight chitosan nanofibers.
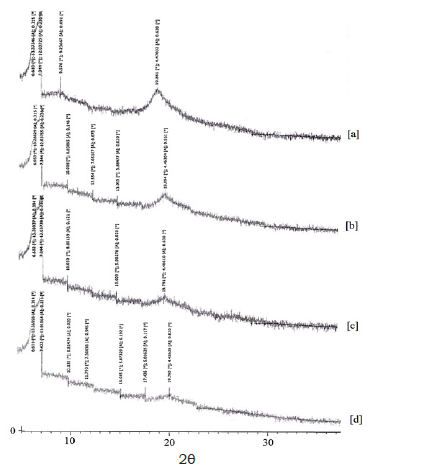
According to the diagrams, high molecular weight chitosan powder is more crystalline than average molecular weight chitosan powder.
The transaction of turning polymer powder into nanofibers (in both high and average molecular weights) has reduced the crystallinity. The crystallinity of high molecular weight chitosan nanofibers comparing average molecular weight chitosan nanofibers isn’t much different. 2θ in powder sample is in between 19.86 and 19.89, which after the transaction to nanofibers has decreased to 19.79; on the other hand, the distance between the plates has increased from 4.46-4.47 to 4.48. In other words, the crystal structure has slightly loosened.
Recovered moisture
Figure 5:Recovered moistures of high and average molecular weight chitosan powder and nanofiber (HF=high molecular weight chitosan nanofibers, MF=average molecular weight chitosan nanofibers, H=high molecular weight chitosan powder, M= average molecular weight chitosan powder).
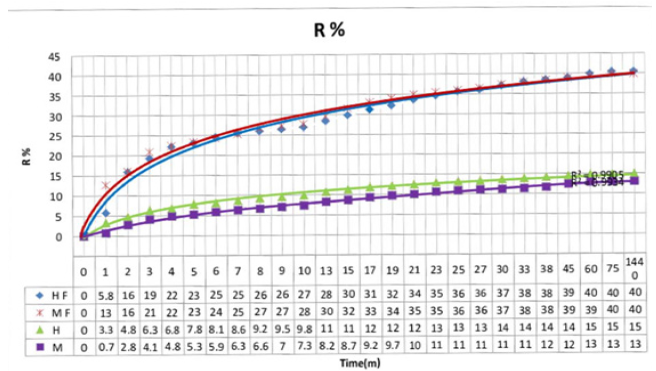
Recovered moistures of both high and average molecular weight chitosan powder and also nanofiber have been compared with each other. The results are presented in Figure 5. Based on Figure 5, high molecular weight and average molecular weight chitosan nanofibers have more adsorption than chitosan powder. This is due to the increase of specific surface area during the transaction of polymer powder into nanofibers. High molecular weight chitosan powder has more adsorption than average molecular weight chitosan powder, which is due to the smaller dimensions of high molecular weight powder than average molecular weight powder. High molecular weight chitosan nanofibers have more adsorption than average molecular weight chitosan nanofibers. This is due to the smaller diameter of high molecular weight chitosan nanofibers compared to average molecular weight chitosan nanofibers.
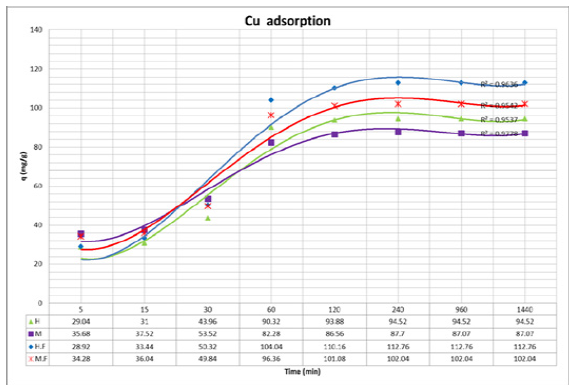
Copper (II) Ion absorption
The adsorption of copper (II) ions by both high and average molecular weight chitosan powder and nanofibers have been compared with each other. The results are presented in Figure 6. Based on Figure 6, high and average molecular weight chitosan nanofibers have more adsorption than chitosan powder. This is due to the increase in specific surface area during the transaction of polymer powder into nanofibers. High molecular weight chitosan powder has more adsorption than average molecular weight chitosan powder. This is due to the smaller dimensions of high molecular weight powder than average molecular weight powder. High molecular weight chitosan nanofibers have more adsorption than average molecular weight chitosan nanofibers, which is due to the smaller diameter of high molecular weight chitosan nanofibers compared to average molecular weight chitosan nanofibers.
Figure 6:Copper (II) ions adsorption of high and average molecular weight chitosan powder and nanofibers (H=high molecular weight chitosan powder, M=average molecular weight chitosan powder, HF=high molecular weight chitosan nanofibers, MF=average molecular weight chitosan nanofibers).
Conclusion
In the case of chitosan nanofibers electro spun with TFA/DCM solvent, high molecular weight chitosan polymer results to thinner nanofibers and better diameter uniformity than average molecular weight chitosan polymer. The optimal spinning solution is 7w% chitosan solution. The diameter of high molecular weight chitosan nanofibers is 128nm. Nanofibers have more specific surface area compared to micro scale fibers which causes more intermolecular interaction. This increases the recovered moisture and the adsorption of heavy metal ions.
References
- Knidri H, Belaabed R, Addaou A, Laajeb A, Lahsini A (2018) Extraction, chemical modification and characterization of chitin and chitosan. International Journal of Biological Macromolecules 120(Pt A): 1181-1189.
- Tang Y, Zhang X, Zhao R, Guo D, Zhang J (2018) Preparation and properties of chitosan/guar gum/nanocrystalline cellulose nanocomposite films. Carbohydrate Polymers 197: 128- 136.
- Vukajlovic D, Parker J, Bretcanu O, Novakovic K (2019) Chitosan based polymer/bioglass composites for tissue engineering applications. Mater Sci Eng C Mater Biol Appl 96: 955-967.
- Muxika A, Etxabide A, Uranga J, Guerrero P, de la Caba K (2017) Chitosan as a bioactive polymer: Processing, properties and applications. International Journal of Biological Macromolecules 105(Pt 2): 1358-1368.
- Fernández Marín R, Mujtaba M, Duman DC, Salha GB, Sanchez MAA, et al. (2021) Effect of Deterpenated Origanum majorana essential oil on the physicochemical and biological properties of chitosan/β-chitin nanofibers nanocomposite films. Polymers 13(9): 1507.
- Lone S, Yoon DH, Lee H, Cheong IW (2018) Gelatin-chitosan hydrogel particles for efficient removal of Hg (ii) from wastewater. Environmental Science: Water Research & Technology 5(1): 83-90.
- Zhang F, Wang B, Jie P, Zhu J, Cheng F (2021) Preparation of chitosan/lignosulfonate for effectively removing Pb (II) in water. Polymer 228: 123878.
- Chakraborty R, Asthana A, Singh AK, Jain B, Susan ABH (2020) Adsorption of heavy metal ions by various low-cost adsorbents: a review. International Journal of Environmental Analytical Chemistry 102(2): 342-379.
- Wadhawan S, Jain A, Nayyar J, Mehta SK (2020) Role of nanomaterials as adsorbents in heavy metal ion removal from wastewater: A review. Journal of Water Process Engineering 33: 101038.
- Qasim SB, Zafar MS, Najeeb S, Khurshid Z, Shah AH, et al. (2018) Electrospinning of chitosan-based solutions for tissue engineering and regenerative medicine. International Journal of Molecular Sciences 19(2): 407.
- Wang W, Bo S, Li S, Qin W (1991) Determination of the Mark-Houwink equation for chitosans with different degrees of deacetylation. International Journal of Biological Macromolecules. 13(5): 281-285.
- Haider S, Park SY (2009) Preparation of the electrospun chitosan nanofibers and their applications to the adsorption of Cu(II) and Pb(II) ions from an aqueous solution. Journal of Membrane Science 328(1-2): 90-96.
- Drits V, Środoń J, Eberl DD (1997) XRD measurement of mean crystallite thickness of Illite and Illite/Smectite: Reappraisal of the kubler index and the scherrer equation. Clays and Clay Minerals 45(3): 461-475.
- British Standards Institution (2021) Method for resistance of fabrics to water absorption (static immersion test). p. 3.
- Igberase E, Osifo P, Ofomaja A (2014) The adsorption of copper (II) ions by polyaniline graft chitosan beads from aqueous solution: Equilibrium, kinetic and desorption studies. Journal of Environmental Chemical Engineering 2(1): 362-369.
© 2022 Niloofar Rafizadeh Zaeem. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






