- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
Electrochemical Properties of Activated Carbon Cloths
Yu M Volfkovich*
AN Frumkin Institute of Physical Chemistry and Electrochemistry of Russian Academy of Sciences, Russia
*Corresponding author:Volfkovich YM, AN Frumkin Institute of Physical Chemistry and Electrochemistry of Russian Academy of Sciences, Moscow, Russia
Submission: July 21, 2022; Published: July 27, 2022

ISSN 2578-0271 Volume 6 Issue5
Opinion
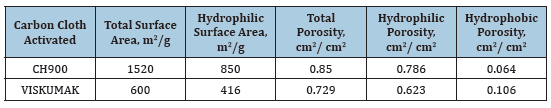
In this work we studied activated carbon cloths (ACC) CH900 from Kuraray (Japan) and VISKUMAK, Russia. To study the porous structure and hydrophilic-hydrophobic properties of these ACCs, the method of standard contact porosimetry (MSCP) was used [1,2]. This method allows not only to study the porous structure of any materials in the widest possible range of pore radii ~ from 1 to 3x105 nm, but also to study their hydrophilic-hydrophobic properties. This method has been recognized by IUPAC [3]. Figure 1 shows SEM micro images for ACC VISKUMAK and ACC CH900. As can be seen from the table, the porous structure of both ACCs includes both hydrophilic and hydrophobic porosity (Table 1).
Figure 1:SEM micro images for a) ACC VISKUMAK and b) ACC CH900
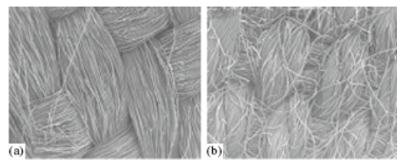
Table 1:The characteristics of the porous structure and hydrophilic-hydrophobic properties of ACC.
It is known that activated carbons contain a large number of ionogenic functional groups (FGs). FG have a significant impact on various properties of activated carbons. Functional group counterions provide surface conduction (SC) for activated carbons. In [4], a technique was developed for measuring surface conductivity (SC) in porous electrodes using activated carbon cloths as an example.
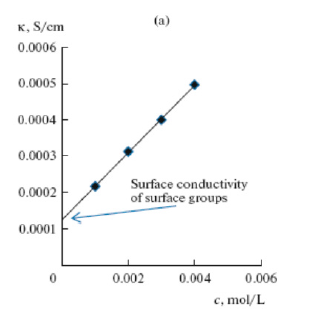
Surface conductivity is the longitudinal (tangential) conductivity of the electric double layer (EDL). On Figure 2 shows the dependence of the electrical conductivity (κ) on the KCl concentrations (c) for the CH900 electrode (a similar dependence was obtained for the WISKUMAK fabric). As you can see, this dependence is almost linear. The value of the surface conductivity was obtained by extrapolating this dependence to the value c=0. Thus, even in pure water, ACC have ionic electrical conductivity due to the presence of a large amount of FGs. It was found that in CH900 the anion exchange and cation exchange capacities are 0.7 and 0.06mmol/g, respectively.
Figure 2:Dependence of the conductivity (κ) on the KCl concentrations (c) for electrode of ACC CH900. Illustration of determination SC by extrapolation.
According to the literature, the specific capacity of porous carbon materials is typically 50-200F/g [5]. However, the specific capacitance values reaching 1100F/g were obtained [5]. In this study, concentrated sulfuric acid with the concentration from 30 to 60% was used as the electrolyte. ACC CH900 was used as the electrode. The measurements were carried out in a wide potential interval from -0.8 to 1.0V (R.H.E). The possibility of carrying out the deep cathodic charging to so negative potentials was provided by the high polarization of hydrogen evolution reaction on carbon materials. Very high specific capacitance 1100F/g were obtained mainly due to the high pseudo capacitance of the reaction of hydrogen intercalation into carbon. This corresponds to the formation of a new compound C6H, which means carbon hydride or hydrogen carbide [6]. Consequently, activated carbon cloth CH900 can be used as high effective supercapacitor electrode.
References
- Volfkovich YM, Bagotzky VS (1994) The method of standard porosimetry 2. Investigation of the formation of porous structures. J Power Sources 48(3): 339-348.
- Volfkovich YuM, Filippov AN, Bagotsky VS (2014) Structural properties of porous materials and powders used in different fields of science and technology. Springer Publisher, London.
- Rouquerol J, Baron G, Denoyel R, Giesche H, Groen J, et al. (2012) Liquid intrusion and alternative methods for the characterization of macroporous materials (IUPAC Technical Report). Pure Appl Chem. 84(1): 107-136.
- Volfkovich YuM, Mikhalin AA, Rychagov AYu (2013) Surface conductivity measurements for porous carbon electrodes. Russian J Electrochemistry 49: 594-598.
- Conway BE (1999) Electrochemical supercapacitors: scientific fundamentals and technological applications. Springer, Germany.
- Volfkovich YuM, Bograchev DA, Mikhalin AA, Bagotsky VS (2014) Supercapacitor carbon electrodes with high capacitance. J Solid State Electrochem 18: 1351-1363.
© 2022 Yu M Volfkovich. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






