- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
Removal of Acid Orange 7 in Textile Wastewater by Homogeneous Ozonation
Dang Thi Thom1,2*, Do Van Manh1,2, Dao Thanh Duong3, Phan Do Hung1, Nguyen Hoai Chau1,2 and Trinh Van Tuyen1
1Institute of Environmental Technology, Vietnam Academy of Science and Technology, Vietnam
2Graduate University of Science and Technology, Vietnam Academy of Science and Technology, Vietnam
3University of Science and Technology of Hanoi, Vietnam Academy of Science and Technology, Vietnam
*Corresponding author:Dang Thi Thom, Institute of Environmental Technology, Vietnam Academy of Science and Technology, 18-Hoangquocviet Str, Caugiay Dict, Hanoi, Vietnam
Submission: June 29, 2022; Published: July 14, 2022

ISSN 2578-0271 Volume 6 Issue 5
Abstract
The textile industrial wastewater with number of dyes including inorganic and organic compounds is very polluted if it is not controlled before discharging into environment. Thus, colour wastewater treatment technology and decolourization efficiency are necessary. The aim of this study is to evaluate the removal efficiency of acid orange 7 (AO7) which has been using at large range in the textile industry by homogeneous ozone process. Decolourization of aquatic solutions containing Acid Orange 7 with initial concentration of 110ppm by using semi-batch reactor where transferred ozone from gas to liquid with initial concentration of 5ppm O3 was studied. The highest decolourization of AO7 solution, about 80%, was achieved at pH3 after 1 hour-treatment time. This efficiency decreased with increasing the initial pH, namely 75%, 60%, 55% and 39% for pH 5, 7, 9, 10, respectively. The decolourization process of AO7 by homogenous ozonation was considered following by the second order kinetics and kinetic constants of the decolourization of AO7 were confirmed the ability of ozonation for decolourization of this dye. The rate constants of decolourization of AO7 were higher at pH 3, 5 with 2.6594M-1s-1, 1.8827 M-1s-1, respectively and lower at pH 7, 9, and 10 with 0.8624M-1s-1, 0.8979M-1s-1 and 0.7777 M-1s-1, respectively. The results obtained shows the homogeneous ozonation capability can be applied to remove AO7 dye.
Keywords:Textile wastewater; Acid orange 7; Homogeneous ozone process; Decolourization
Introduction
Dyes are used with a large amount of the textile industry and thus pollutants from dyes discharge into environment day by day if they are not controlled. It is assumed that there are about 10.000 commercial dyes used in the textile industry with the biggest number about 60% of azo dyes [1,2]. Some industries such as textile, plastic, leather, paper industries as additives, especially the textile industry consumed the largest dyes in manufacturing [1,3,4]. Thus, textile wastewaters are one of the most polluted wastewaters due to their characteristics include mixing contaminants. Moreover, over 7x105 tonnes of dyes are produced annually worldwide. It is predicted that about 10-15% of these dyes are discharged in effluents during dyeing processes [5]. Therefore, environmental problems of wastewater discharges are mainly focused of the textile industry.
In Vietnam, the textile industry plays an important role in economy development with regards to production sources of labour force employment and of export products. It accounts for 10% of employed industrial labours and 14.5% of the country’s total export turnover. However, the textile wastewater in from 25 textile enterprises in Vietnam has been heavily polluted due to a wide range of COD concentrations from 245-11,150mg/L and amount of chemicals from dyes which are from different organic and inorganic compounds [6]. The ratio of BOD5/COD found is low (around 0.2-0.5) which demonstrates this wastewater is considered as being very hard to degrade by biological methods and other conventional methods due to BOD5 is less than COD so far.
Textile wastewater pollution was studied in term of COD, BOD, TSS, TDS, TOC, heavy metals in which dyeing organic compounds was focused. In another side, many dyes are toxic and easy to affect to aquatic communities and ecosystem without treatment, thus they are possibly follow-on food chain leading to human beings and environmentally safe [7]. Thus, characteristics of textile wastewater are required an adaptive resolution of removing these contaminants.
For dye treatment, some conventional processes are used such
as physico-chemical treatment methods including coagulation,
and flocculation, ion-exchange or ultra-filtration and reverse
osmosis and biological treatment methods [1,3,8,9]. However,
these methods are limited in colour removal due to existence of
recalcitrant compounds of dyeing textile wastewater and excess
sludge production during treatment processes. Recently, advanced
oxidation processes (AOPs) become more effective and are
commonly used in alternative treatments by presence of strong
oxidants such as ozone and hydrogen peroxide [8]. Ozone is a
powerful oxidizing agent and ozonation process has been developed
for wastewater treatment. Many research have illustrated that
ozonation process has bring high effectiveness in removing dyes in
textile wastewater [10-15]. Especially the ozonation of azo dyes has
been interested in research and application due to
over 60% azo dyes have been used in the textile industry.
azo-dyes are difficult to bio-degrade by conventional treatment
methods.
ozone is powerful reactive with the azo (-N=N-) double bond.
Therefore, decolourization of dyes can be easily removed in wastewater by ozonation [15]. The ozonation is not only effective in removing the colour but also so excellent in reducing COD and TOC [4].
In this paper, acid orange 7 (AO7), an azo dye is selected as pollutant to investigated by the ozonation because it is difficult to biodegrade. In some previous research, acid orange 7 is also studied by catalyst in the hydrogen peroxide oxidation and by the combination of ozone and hydrogen peroxide in a hollow fiber membrane reactor [16-18]. However, those results are limited due to processes takes long time and not high efficiency for removing AO7 in their experimental conditions. Therefore, study on decolourization efficiency of AO7 by homogeneous process using ozone as an oxidant and in various conditions of pH and reaction time is investigated.
Materials and Methods
Materials
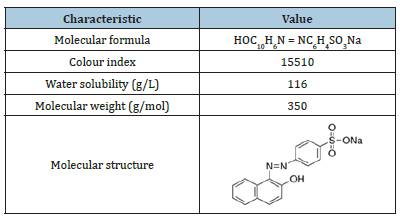
Acid orange 7 (AO7) was purchased from Merck, Germany, chemical formula is C16H11N2NaO4S with characteristics of acid orange 7 on Table 1. Sodium hydroxide (NaOH) 1M, acid sulfuric (H2SO4sub>) 1M from Merck-Germany was used to adjust pH in the different conditions. Glycine (C2H5N2) was used to quit reaction of ozone in dye analysis during experimental process.
Table 1:Characteristics of acid orange 7.
Experimental set-up
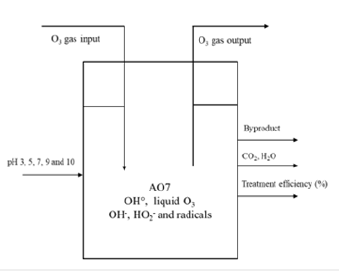
Oxygen is pumped from oxygen cylinder, pass through ozone generator to enrich ozone liquid into column batch reactor. Ozone in the liquid is formed in the batch reactor with 2L capacity with different pH conditions from 3 to 10. Ozone gas of the output can pass though the destructor which used to treat ozone gas before discharging into environment. After ozone generation, turning off ozone generator, opening circulation pump and adding dye compounds to mix frequently in the reactor. Air diffuser inside batch reactor can create small bubble to mix the gas transferred well in the liquid.
Parameters in process are evaluated in the column batch reactor of 2L after liquid ozone is transferred from gas O3 in pure water matrix in reaction with AO7 added in different conditions. In each process, the ozone generation in pure water matrix is performed at least 30 minutes to optimize ozone concentration in the liquid. Liquid O3 concentration in reactor is saturated after 30 minutes of generation. The dye component of AO7 is added immediately into the batch reactor after stopping O3 generation and initial ozone concentration is determined. The experiments are conducted in the range of pH from 3 to 10 for removing initial acid orange 7 concentration of 110ppm during one hour of reaction time. Investigation of decolouration for AO7 by ozonation is studied with reactions possibly happening in the experimental system as follows on Figure 1.
Figure 1:Diagram of experimental system.
Analytical methods
Ozone in liquid is measured under Indigo Carmine method at wavelength of 595nm by UV-visible spectrophotometer (UH5300 spectrophotometer, HITACHI-Japan) [19]. Acid orange 7 is determined by spectrophotometer method at wavelength of 481nm by UV-visible spectrophotometer (UH5300 spectrophotometer, HITACHI - Japan). Concentration of AO7 is calculated from calibration curve at 481nm. All parameters are monitored versus experimental time and taken out with approximately 5ml of volume from batch reactor of 2L by syringe of 10ml.
All experiments are implemented at least in one hour and based on obtained analysis data, the residual concentrations of dyes are calculated. Efficiency of the process for colour removal is examined by the formula:
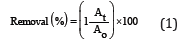
Where: and are concentrations of dye at time t and 0, respectively.
The efficiency and feasibility of removing AO7 wastewater is appreciated by the kinetics of the ozonation. When the ozone concentration is assumed to reach the interface of the stationary state, the oxidation rate with respect to the concentration of the organic substance as AO7 compound is determined.
Thus, approximate kinetics for colour removal of dye solution by ozonation can be reported. The oxidation rate considered as pseudo-first order kinetics of colour removal is investigated by the following rate equation:
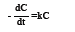
Where dC/dt is the rate of change of dye concentration. Negative sign indicates that concentration of dye decreases with time t, k is the rate constant (min-1 or s-1) and C is the concentration of dye at time t. If the initial concentration of dye at time t=0 is C0 and at some later time t, the concentration has fallen to C, then the integration of equation (2) between t=0 and t=t gives as bellows:
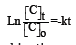
Thus, a first-order kinetic constants can be determined by plotting a graph of ln[C] vs. time t, a straight line is produced with slope -k.
If this process happened by the second rate kinetics, the change of colour concentration versus time was expressed by the following equation:
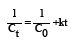
Where: C0 (M): initial AO7 concentration at time=0
Ct (M): AO7 concentration at time t (s)
Hence, second-order kinetic constants can be determined by plotting a graph of[1/Ct= 1/C0] vs time t, a straight line is produced with slope k.
Result and Discussion
Decomposition of ozone concentration in removal of OA7
Ozone generation process was performed in reactor of 2L at room temperature. Sodium hydroxide (NaOH 1M) and acid sulfuric (H2SO4 1M) were added into the column batch reactor to obtain different pH values (3; 5; 7; 9; 10) in experiments. Figure 2 revealed the amount of produced ozone after ozone generation with different set-up pH and initial liquid ozone concentration was determined at 0s of starting time. Experiments were conducted for removing AO7 in reactor of 2L after ozone transferred from gas to liquid. Iinitial ozone concentration in the range from 5.8-6.5ppm at pH from 3-7 is introduced for experiments for removing initial AO7 concentration of 110ppm. Ozone was consumed quickly and immediately in reaction with orange acid 7 at pH3 and pH5. Ozone is consumed speedily, leading to almost ozone concentration declined to under 0.8ppm especially at pH3, ozone concentration declined to 0.22ppm only some starting minutes and at this pH, almost ozone concentration was consumed over in reaction to remove orange acid 7 in the ozone decomposition.
Figure 2:Ozone concentration decomposition in decolourization of AO7.
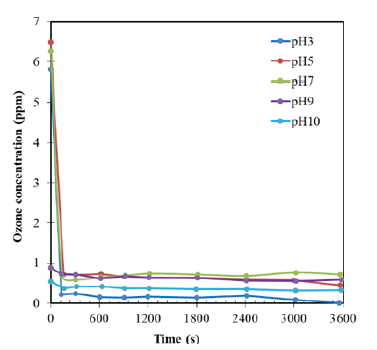
At pH9 and 10 of the alkaline conditions, ozone decomposed immediately in reactor during ozone generation. These obtained results confirmed that almost ozone was converted to OH° at pH 9 and 10 (Figure 2) and ozone was decomposed quickly at high pH to form hydroxyl radicals as OH° [20]. Thus, at pH9, initial ozone concentration gets 0.95ppm before reaction with AO7 110ppm added and then O3 concentration decreased slowly from 0.95ppm to 0.8ppm during one hour of experimental manipulation. At pH10, ozone concentration changed limitedly from 0.5ppm to 0.35ppm at the end of experiment after adding AO7 110ppm. Thus, it was assumed that, OH° radical played an important role at the alkaline condition for removing orange acid 7 in these cases and ozone decomposition has produced OH° radicals during O3 generation
in the liquid. Investigated data in this study were adaptive with previous authors who studied on mechanism and kinetics of ozone decomposition [21-23]. Ozone decomposes partly in OH° radicals. When the pH value increases, the formation of OH° radicals increases. In a solution with a high pH value, there are more hydroxyl ions presented, see formulas below. These hydroxyl ions act as an initiator for the decay of ozone:
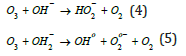
The anions in reaction 1 that are produced into reaction 2 can introduced other reactions with ozone, generate more OH° radicals.
Removal efficiency of homogenous ozone process for AO7
After ozone generation in 2 litters of pure water in the reactor at different initial pH conditions, AO7 was added to obtain with fixed initial concentration of 110ppm from stock solution of 10g/l. The efficiency of AO7 removal at pH 3; 5; 7; 9 and pH 10 are almost 80%; 75%, 60% and 39% respectively in one hour of reaction time (Figure 3). AO7 concentration reduced from 110ppm to 17.82ppm at pH 3 to 22.59ppm at pH5 and almost to 43ppm at pH7, to 45ppm at pH 9 and lightly change to 70ppm at pH10. In first 10 minutes, the colour removal abilities at pH 3 and pH 5 were really good, nearly 45% of initial AO7 concentration was degradable. However, at higher pH values, such as pH7 and pH9, the removal efficiencies were much lower, nearly 20%. After that, rate of colour removal increased gradually versus time which corresponded to the decrease in ozone concentration during process. These results demonstrated clearly the high pH conditions were not convenient for treating AO7 by ozonation process. Therefore, conditions of initial pH 3 and 5 were suitable for removal of AO7 with only low initial ozone concentration of from 5.8-6.5ppm (Figure 3&4).
Figure 3:Efeect of pH in discolourization of AO7 versus time.
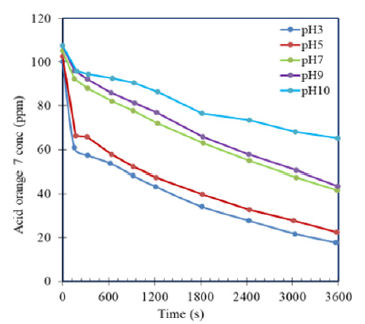
Figure 4:Efficiency of removing AO7 versus time.
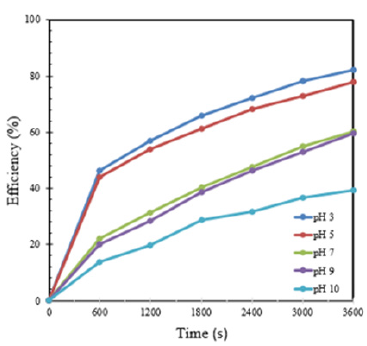
In ozone generation in the batch reactor using pure water, pH 3 is considered as the best condition to degrade AO7, which leads to the ratio of residual and initial concentration of that dye at acidic condition was lowest versus time during process. However, this value was nearly the same condition at pH5. Besides that, at pH7 and 9, the lines of the ratio of residual and initial concentrations were nearly similar and that ratio of pH 10 was also investigated on Figure 5. Hence, decolourization of AO7 is better in acidic conditions at pH3 and pH5.
Figure 5:Ratio of residual and initial AO7 concentration during homogenous ozone process.
Ozonation kinetics
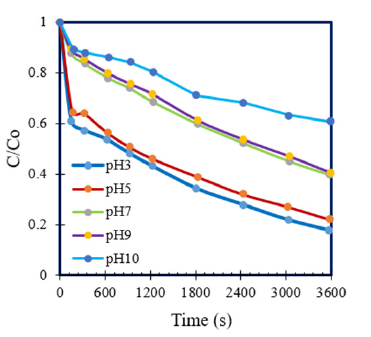
The obtained data showed that, this degradation of AO7 was predicted as the pseudo first order kinetic model on Figure 6 and the second order kinetic model on Figure 7 where pseudo first kinetic constant (k, s-1) and second kinetic constant (k, M-1s- 1) depend on time running and experimental conditions of initial pH. Rate constants of reaction (k) of AO7 degradation by the pseudo first order and second order kinetics at different pH were summarized in Table 2. The different linear functions related to the pseudo first order and the second order kinetics were used to determine R2 coefficients. The results were showed that, values of R2 coefficients of the second order kinetics were bigger than those of the pseudo-first order kinetics (Table 2). As can be seen, these data confirmed that AO7 degradation process by homogeneous ozonation in different pH from 3 to 10 had the fitness trend of the second order kinetics.
Figure 6:First order kinetics of AO7 decolourization.
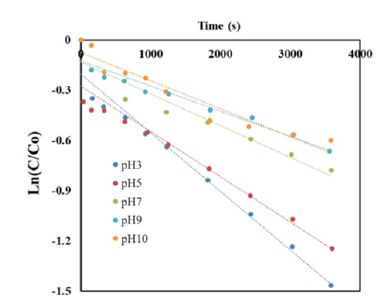
Figure 7:Second order kinetics of AO7 decolourization.
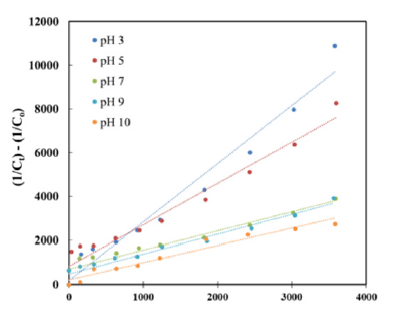
Table 2:Kinetic constants of AO7 decolourization at various pH and initial AO7 concentration of 110ppm (k, kinetic constant).
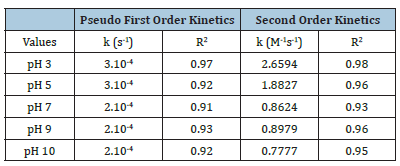
The mechanism performed at pH 3 and pH 5 was better with the kinetic constants 2.6594M-1s-1 and 1.8827M-1s-1, respectively. The kinetic constants at pH 3 and pH 5 get higher from 2.1 to 3.4 times than the rate constants of pH 7, pH 9 and pH 10 (0.7777 - 0.8979M-1s-1) with R2 coefficient values in the range from 0.93 to 0.98. In comparison with the degradation of Acid Orange 7 by a heterogeneous catalytic wet hydrogen peroxide process, this complete decolourization was reached 4 hours of reaction and even using less initial concentration of AO7 (5.10-5 to 1.9.10-4M) [16] and the decolourization kinetics followed pseudo-half-order-kinetics [17].
Conclusion
Decolourization of AO7 with experimental conditions of pH were investigated in this study, homogeneous ozonation at pH 3 showed to be more effective in terms of decolourization of this AO7 dye. The removal efficiency for AO7 were 80%; 75%, 60% and 39% at pH 3, 5, 7, 9 and 10 at which pH 7 and 9 this degraded efficiency was nearly similar during 60 minutes of reaction time. Therefore, it was indicated that only needed low initial ozone concentration from 5.8-6.5ppm at pH 3 and pH 7 were also enough to remove more than 75%-80% this dye in 60 minutes of reaction time by homogeneous ozone process. Consequently, the decolourization kinetics of AO7 dye fit the second order kinetics as investigated data in aqueous solution at pH 3, 5 got kinetic constants 2.6594M- 1s-1, 1.8827M-1s-1, respectively and at pH 7, 9 and 10 got kinetics constants 0.8624, 0.8979 and 0.7777M-1s-1, respectively in reliable R2 coefficients.
Acknowledgement
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 105.99-2018.18. The authors acknowledged gratefully to this financial support.
Credit Authorship Contribution Statement
Dang Thi Thom, Do Van Manh, Dao Thanh Duong, Phan Do Hung, Nguyen Hoai Chau and Trinh Van Tuyen outline the research,plan the experiments, prepare data and finish the draft manuscript. Dang Thi Thom and Dao Thanh Duong do experiments to obtain data of the research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Fahmi SA, Abidin CZA, Mohd Makhtar SNN, Rahmat NR, Ahmad R, et al. (2016) Decolourization and cod reduction of textile wastewater by ozonation in combination with biological treatment. Int J Automot Mech Eng 13: 3141-3149.
- Muniyasamy A, Sivaporul G, Gopinath A, et al (2020) Process development for the degradation of textile azo dyes (mono-, di-, poly-) by advanced oxidation process-Ozonation: Experimental & partial derivative modelling approach. J Environ Manage 265: 11039.
- Abidin CZA, Fahmi, Ong SA, Ahmad R, Sabri SN, et al. (2017) Treatment of azo dye Acid Orange 52 using ozonation and completed-mixed activated sludge process. IOP Conf Ser Mater Sci Eng 206: 012088.
- Zulzikrami Azner Abidin C, Fahmi MR, Soon An O, Makhtar SNNM, Rahmat NR, et al. (2015) Decolourization of an azo dye in aqueous solution by ozonation in a semi-batch bubble column reactor. Science Asia 41(2015): 49-54.
- Najafi H, Movahed HR (2009) Improvement of COD and TOC reactive dyes in textile wastewater by coagulation chemical material. Afr J Biotechnol 8(13): 3053-3059.
- WWF Report (2018) Textile and garment sector in Vietnam: Water risks and solutions.
- Wijannarong S, Aroonsrimorakot S, Thavipoke P, Kumsopa C, Sangjan S, et al. (2013) Removal of reactive dyes from textile dyeing industrial effluent by ozonation process. APCBEE Procedia 5: 279-282.
- Sharma S, Buddhdev J, Patel M, Ruparelia JP (2013) Studies on degradation of reactive red 135 dye in wastewater using ozone. Procedia Eng 51: 451-455.
- Arslan S, Eyvaz M, Gürbulak E, Yüksel E (2016) A review of state-of-the-art technologies in dye-containing wastewater treatment-the textile industry case. In: Kumbasar EPA, Körlü AE (Eds.), Textile Wastewater Treatment.
- Poznyak T, Colindres P, Chairez I (2007) Treatment of textile industrial dyes by simple ozonation with water recirculation. J Mex Chem Soc 51(2): 81-86.
- Wu CH, Kuo CY, Chang CL (2007) Decolorization of AZO dyes using catalytic ozonation. React Kinet Catal Lett 91: 161-168.
- Palit S (2009) Ozonation of direct red-23 dye in a fixed bed batch bubble column reactor. Indian J Sci Technol 2(10): 14-16.
- Khan H, Ahmad N, Yasar A, Shahid R (2010) Advanced oxidative decolorization of red cl-5b: effects of dye concentration, process optimization and reaction kinetics. Pol J Environ Stud 19(1): 83-92.
- Turhan K, Turgut Z (2009) Decolorization of direct dye in textile wastewater by ozonization in a semi-batch bubble column reactor. Desalination 242(1-3): 256-263.
- Tizaoui C, Grima N (2011) Kinetics of the ozone oxidation of Reactive Orange 16 azo dye in aqueous solution. Chem Eng J 173(2): 463-473.
- Herney Ramirez J, Silva AMT, Vicente MA, et al (2011) Degradation of acid orange 7 using a saponite-based catalyst in wet hydrogen peroxide oxidation: Kinetic study with the Fermi’s equation. Appl Catal B: Environ 101(3-4): 197-205.
- Zhang H, Wang W (2011) Oxidation of C.I acid orange 7 with ozone and hydrogen peroxide in fiber membrane reactor. Chem Eng Commun 198(12): 1530-1544.
- Muthukumar M, Sargunamani D, Senthilkumar M, Selvakumar N (2005) Studies on decolouration, toxicity and the possibility for recycling of acid dye effluents using ozone treatment. Dyes and Pigments 64(1): 39-44.
- Bader H, Hoigné J (1981) Determination of ozone in water by the indigo method. Water Research 15(4): 449-456.
- Mandake MB, Dave DS, Goswami DG, Yeram SS (2016) Removal of COD and color of carmine dye by photocatalytic ozonation with effect of zinc oxide catalyst. International Journal of Research in Advent Technology 4(6): 46-53.
- Turhan K, Durukan I, Ozturkcan SA, Turgut Z (2012) Decolorization of textile basic dye in aqueous solution by ozone. Dyes Pigments 92(3): 897-901.
- Tomiyasu H, Fukutomi H, Gordon G (1985) Kinetics and mechanism of ozone decomposition in basic aqueous solution. Inorg Chem 24(19): 2962-2966.
- Staehelin J, Hoigne J (1985) Decomposition of ozone in water in the presence of organic solutes acting as promoters and inhibitors of radical chain reactions. Environ Sci Technol 19(12): 1206-1213.
© 2022 Dang Thi Thom. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






