- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
Physicochemical Characteristics and Biological Activities of Chitosan-O-Kojic Acid Conjugate Polymer
Xiaoli Liu1*, Wancui Xie2, Xihong Yang2, Xiaobei Zhan3, Wenshui Xia1
1State Key Laboratory of Food Science and Technology, China
2College of Marine Science and Biological Engineering, China
3School of biological engineering, China
*Corresponding author: Xiaoli Liu, State Key Laboratory of Food Science and Technology, China
Submission: September 12, 2019; Published: September 20, 2019

ISSN 2578-0271 Volume5 Issue4
Abstract
The main objective of this work was to evaluate the physicochemical characteristics and biological activities of chitosan-o-kojic acid conjugate polymer (COS-O-KA) due to the conjugation of chitosan with kojic acid. The characteristics of water solubility, pH stability as well as hemolysis assay and cytotoxicity tests in vitro, zeta potential, and animal toxicity studies were investigated. The antioxidant activities in vitro were evaluated by DPPH•, •OH, •O2−, ABTS•+, and Fe2+ chelating assays, which revealed that conjugation notably enhanced the antioxidant activities relative to free COS and KA. Owing to its noncytotoxicity and low hemolysis, COS-O-KA might be considered a promising material for antioxidant applications.
Keywords: Chitosan oligosaccharide; Kojic acid; Antioxidant activity
Introduction
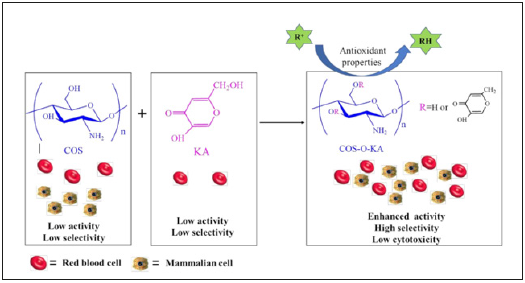
There are increasing evidence demonstrates that excessive amount of reactive oxygen species (ROS) and oxygen-derived free radicals (OFR) can destroy cellular components and may cause many diseases such as cancer, diabetes, aging, and atherosclerosis [1-4]. Hence, it is extremely important and necessary to develop and utilize safe and effective materials with antioxidant activity to prevent human body being damaged by free radicals [5,6]. Of late, numerous natural polysaccharides and their derivatives have been given evidence of displaying effective antioxidant activities and being developed as novel potential antioxidants [7-9]. Among various renewable natural polysaccharides, especially, chitosan oligosaccharide (COS), a promising and inexpensive resource for the fabrication of versatile biopolymeric material, is under intense research [10,11]. Due to its excellent anticancer, antimicrobial properties [12], intrinsic biodegradability, and biocompatibility, COS has been widely applied in several fields, such as pharmaceutical, biotechnology, cosmetics, textiles, effluent treatment, and agriculture [13-15]. Despite such desirable characteristics, its practical utility is limited due to the relatively lower antioxidant capacity compared with that of traditional antioxidants, such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) [16]. The presence of three useful active functional groups (a primary amine at C2, primary hydroxy at C6 and secondary hydroxy at C3 positions) in COS imparts unique chemical behavior and allows it to be readily modified to further augment the antioxidant activity [17-19]. Therefore, in recent years, research workers are continuing their unremitting efforts to design, prepare, and test the new, nontoxic, biocompatible, and low costing bioactive materials. The hydroxy groups of COS are the main sites for modification and a variety of 44 derivatives were synthesized through grafting reaction [20], quaternary reaction [21] and carboxymethyl reaction [22]. Among the methods of modification of COS, grafting reaction is one of the dramatic approaches, which can afford a great variety of monomers of active functional groups to COS chain by covalently bonded [23,24]. However, the issue of relatively low selectivity of some grafting COS-based antioxidant materials, leading to poor biocompatibility, cytotoxicity, and hemolysis, needs to be resolved. Kojic acid (KA, 2-hydroxymethyl-5-hydroxy-4H-pyran- 4-one), a fungal metabolite, is frequently used as a browning inhibitor in the food industry [25,26]. KA at concentrations in the range 1.6-50μg/mL did not effect, on the viability of normal skin cell and skin cancer cells during a 72-hours incubation [27]. Chlorokojic acid (Cl-KA) and further halo-derivatives of KA, which act as efficient antioxidants and as ligands for nucleophilic and electrophilic substitutions, have been synthesized and applied in food, medical, cosmetic, and chemical industries [28,29]. Besides, they are well-known as potent bidentate metal chelators [30]. Partially, kojic acidchitosan- tri-polyphosphate nanoparticles and kojic acid-loaded chitosan coated magnetic nanoparticles (KA-CS-MNPs) enhanced the antibacterial activity of KA against both Gram-positive and Gram-negative, and showed good bio adhesive properties [31,32]. Inspired by biological activity of COS and KA, we developed a simple approach for preparing COS-O-KA polymer, and COS-O-KA was prepared based on our previously reported process [13]. The reaction conditions, yield, degree of substitution (DS), numberaverage molecular weight (Mn), weight-average molecular weight (Mw), and polydispersity index (PID) were shown in Table 1. Furthermore, the water solubility, pH stability, cytotoxicity tests in vitro, zeta potential, antioxidant activity and animal toxicity were systematically evaluated. Figure 1 shows the reaction scheme for the preparation of COS-O-KA and its bioactivity. We postulated that the combination of COS and KA, with synergistically diverse modes of action, could facilitate further derivatization to achieve excellent antioxidant efficiency and low cytotoxicity (Table 1) (Figure 1).
Table 1: Reaction conditions for COS with KA, Yield, DS, Water solubility, Mn, Mw, and PID for the COS-O-KA1-3.
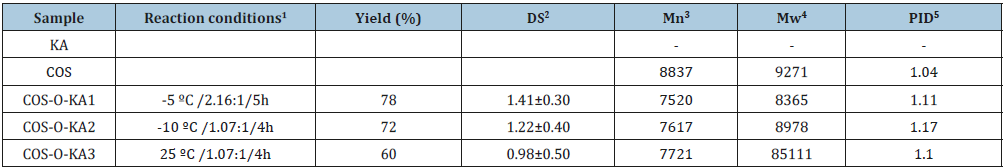
Reaction temperature/Molar ratio (Chlorokojic acid to N-benzylidene COS)/Reaction time.
Values are expressed as mean±standard deviation.
Mn: number-average molecular weight.
Mw: weight-average molecular weight.
PID: polydispersity index.
-: is no testing.
Figure 1:Reaction scheme for the preparation of COS-O-KA and its bioactivity.
Materials and Methods
Materials
COS (8828 Da; 90% deacetylation degree) was sourced from Zhejiang Jinke Biochemistry Co.Ltd, (Zhejiang, China). COS-OKA was prepared based on our previously reported process [13]. KA was obtained from Wuhan Weisheng Biochemical Co. Ltd., (Wuhan, China). Cl-KA was prepared based on the Uher’s technique with minor modifications [33]. COS-O-KA was prepared based on our previously reported process. DS was defined as the mol ratio of bonded kojic acid per mol of glucosamine calculated from the original mass of COS. The deacetylation degree (DD) and DS were calculated from the integrals of 1H NMR spectrum. The average molecular weight (Mw) of COS and COS-O-KA1-3 were calculated using gel permeation chromatography (GPC). 2,20-Azinobis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) was purchased from Honeywell International Inc, USA. Nitro blue tetrazolium (NBT) and 2,2-Diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Human aortic smooth muscle cells (HASMC CC-2571) was purchased from Saiqi Biological Engineering Co. Ltd., (Shanghai, China). Riboflavin, methionine, Tris-HCl buffer solution (0.05 mol/L Tris, 0.10 mol/L NaCl, pH=7.4), N-methyl piperazine, benzaldehyde, pyridine, potassium ferricyanide, ethylene diamine tetraacetic acid (EDTA), N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), thionyl chloride (SOCl2), phosphate-buffered saline (PBS), acetone, ethanol, methanol, and deuterium oxide (D2O) were purchased from Sinopharm Chemical Reagent Co., Ltd., (Shanghai, China). All other chemicals and reagents were of analytical grade.
Determination of pH stability
Five g of the samples (COS, COS-O-KA1, COS-O-KA2, and COSO- KA3) were dissolved in 1% acetic acid (100 mL). The COS-OKA1- 3 were prepared under different reaction conditions, and with different DS, Mn, and PID, which shown in Table 1. One M NaOH solution was added gradually to the solutions. Determination of pH stability of the solutions were examined by transmittance at 600nm by using UV1000 UV-Vis spectrophotometer (Techcomp Ltd., China). [34,35].
In vitro hemolysis assay
In brief, 10mL of freshly collected blood from a 26-year old healthy male donor, who gave signed informed consent, was centrifuged at 580×g for 10min. The separated erythrocytes (RBC) were diluted to 5% (v/v) after washing thrice with Tris (10mM) and Tris buffer (150mM, pH = 7.2). Several concentrations (0.5-2.5mg/ mL) of sample solutions were added into the RBC stock (1:1, v:v) in 96-well microplates, followed by incubation in a shaking incubator at 37 °C for 1h. Subsequent to centrifugation for 10min at 180×g, the supernatants (100μL) were added to fresh microplates and mixed with Tris buffer (100μL). Triton X-100 (0.1% in Tris buffer) which is able to lyse RBC completely was used as positive control, while Tris buffer was used as negative control. The hemolysis (%) was estimated by absorbance at 541nm with a Spectra Max M5 microplate reader (Molecular Devices, Pennsylvania, USA) using the equation:
Hemolysis (%) = [(As – Ab)/(At – Ab)] ×100% Eq(2)
Where As, At and Ab are the absorbance value for the samples (COS, KA, and COS-O-KA1-3), positive control (Triton X-100) and negative control (Tris buffer), respectively. The procedures were carried out in accordance with the protocols of Jiangnan Univerisity’s Institutional Medical Ethics and Use Committee (JN. No20190228c0360425[8]).
In vitro biocompatibility assay
The cell viability was investigated through 3-(4,5-dimethyl-2- thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) protocol [36]. Human aortic smooth muscle cells (HASMC CC-2571) were added to a 96-well plate (1 ×105 cells/well), diverse concentrations of COS-O-KA1-3 (5,10,15,20 and 25mg/mL) 200μL were added and were incubated at 37 °C for specific times. Subsequently, MTT (20μL) was added to the wells, followed by incubation for 4h, and centrifugation at 720×g for 5min. Next, the supernatant was replaced with DMSO (100μL), the plate was shaken for 15min and the absorbance was recorded at 490nm.
Zeta potential measurement
Zeta potential was determined for all the samples (COS,KA and CO-O-KA1-3) at pH 2,3,4,5,6,7,8,9,10,11 and 12 by means of a Zeta PALS Analyzer (Brookhaven Instruments Corp., Austin, Texas, USA). The calibration of instrument was done with a standard of –50±5 mV at 25 °C according to the operation instructions.
DPPH• scavenging activity
Briefly, different concentrations of COS, KA, and CO-O-KA1-3 (0.5-2.5mg/mL) were transferred to 96-well plates (50μL/well) and DPPH (200μL, 0.4mM) was supplemented to the wells [37]. The mixtures were vigorously shaken and subsequently maintained in the dark for 30min. Absorbance at 516nm was recorded. The DPPH• scavenging capacity (SA) was estimated using Eq. (3):
SA (%) = [1-(A1-A2)/A0] ×100 Eq. (3)
Where A0 (water instead of sample), A1, and A2 are the absorbances of the control, sample, and background (water in place of DPPH), respectively.
OH• scavenging activity
The •OH scavenging activity of antioxidants were estimated according to an earlier method [5]. The mixture containing 1mL COS, KA, and CO-O-KA1-3 (0.5-2.5mg/mL of samples in distilled water), 2mL FeSO4 (9mM), and 2mL H2O2 (0.3%) in salicylic acid-ethanol solution was incubated at 37 °C for 30min, and subsequently cooled down to measure their absorbance at 510nm against reagent blank. The •OH scavenging activity (SA) was measured using Eq. (4).
SA (%) = [1-(A1-A2)/A0] ×100 Eq. (4)
Where A0, A1, and A2 are the absorbances of the control, sample, and background, respectively.
•O2− scavenging activity
Reaction was accomplished in a mixture containing sodium phosphate buffer (50mM, pH 7.8), NBT (70μM), methionine (13mM), EDTA (100mM), riboflavin (10Mm), and antioxidant samples (1 mL,0.25-2.5mg/mL) that according to an earlier method [3]. The absorbance was recorded at 560nm after incubated at room temperature for 10min. The •O•O2− scavenging activity (SA) was measured using Eq. (5).
SA (%) = [1-(A1-A2)/A0] ×100 Eq. (5)
Where A0, A1, and A2 are the absorbances of the control, sample, and background, respectively.
ABTS•+ scavenging activity
The ABTS•+ scavenging capacity was determined according to an earlier method [24,38], with some modification. Briefly, ABTS (7mM) was reacted with potassium persulfate (4.95mM) and the mixture was kept in the dark at 25 °C for 12h. Then, each sample (0.5-2.5mg/mL of samples in distilled water) and ABTS working solution (200μL) were mixed together, and the absorbance was monitored at 735nm. The ABTS•+ scavenging capacity (SA) was measured using Eq. (6).
SA (%) = [1-(A1-A2)/A0] ×100 Eq. (6)
Where A0, A1, and A2 are the absorbances of the control, sample, and background, respectively.
Fe2+ chelating activity
The Fe2+ chelating capacities of COS, KA, and CO-O-KA1-3 were determined by means of Topal’s technique with some modifications [4]. Initially, ferrozine solution (0.2mL, 5mM), FeCl2 solution (0.05 mL, 2mM), water (2.75mL) and samples (1.0mL, 0.5-2.5mg/mL) were mixed together. After 10min incubation at 25 °C, UV-Vis spectra were recorded at 562nm. The chelating capacity (CA) was measured using Eq. (7).
CA (%) = [1-(A1-A2)/A0] ×100 Eq. (7)
where A0, A1, and A2 are the absorbances of the control, sample, and sample with deionized water instead of FeCl2 solution, respectively.
Animal toxicity study
Forty healthy female SLAC KM mice (6-8weeks old, 20.0±2.0 g) were randomly segregated into three drug administration groups and a control group, with 10 mice in each group. COS-O-KA1-3 solution in sterile PBS (100μL) was injected through the tail vein to the drug administration groups at the doses of 25,18 and 6mg/kg in the high, medium and low dose groups, respectively. The treated mice were monitored 7 days after administration. The control mice were intravenously administered with 9mg/mL NaCl solution. The mice were handled in accordance with Jiangnan Univerisity’s Institutional Animal Care and Use Committee protocols (JN. No20190228c0360425[8]).
Results and Discussion
Determination of pH stability
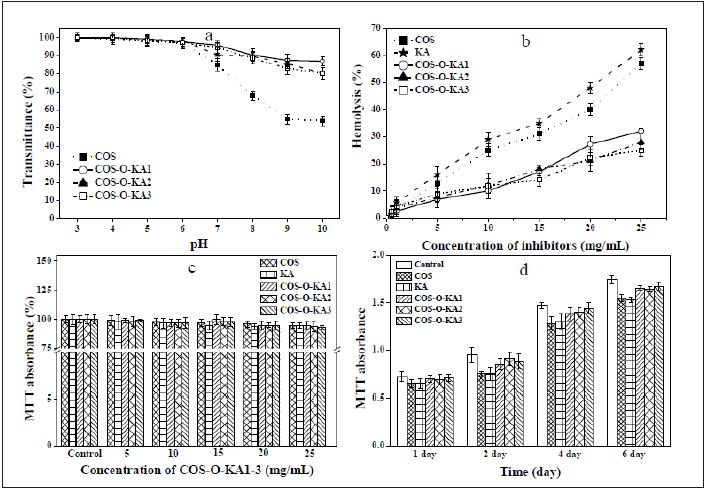
Figure 2a shows the stability of all the samples at different pH values. Increasing the pH of aqueous solution from 3.0 to 10.0, COS-O-KA1 exhibited the highest solution stability at higher pH values (pH=10, 86.94%). As the pH was increased from 7.0 to 9.0, the transmittance of the COS solution decreased rapidly. On the contrary, the transmittance of COS-O-KA1-3 solutions slowly declined. These results signified that COS-O-KA1-3 polymer had superior pH stability than COS in basic conditions (Figure 2).
Figure 2:a: pH stability of COS and COS-O-KA1-3; b: Hemolytic activity and of COS, KA, and COS-O-KA1-3 at different concentrations. c: MTT of HASMC cultured in a series of concentrations of COS-O-KA1-3 for 1h. d: HASMCs cultured with 15mg/mL COS-O-KA1-3 from 1 to 6 days.
In vitro hemolysis assay
At 15mg/mL, COS-O-KA1-3 exhibited less than 20% hemolytic percentage, whereas the hemolytic activities of COS and KA were more than 30% at the same concentrations (Figure 2b). At 25mg/ mL, the hemolytic activity was less than 35% for COS-O-KA1-3, while the activities of COS and KA were greater than 50%. The percentages of COS-O-KA1, COS-O-KA2, and COS-O-KA3 ranged from 25% to 32% with slightly increased hemolytic activity with increasing DS. Hence, comparing COS with COS-O-KA1-3, grafting with KA reduced the hemolytic activity and COS-O-KA1-3 shows excellent non-hemolysis as well as potent antimicrobial and antioxidant activity [13, 39]. These results signified an exceptionally high selectivity of COS-O-KA1-3 for microbes over human RBC.
In vitro biocompatibility study
In the MTT assay, HASMC were viable in the presence of diverse concentrations of COS-O-KA1- 3 for 1h and there was no decline in HASMC viability below the concentration of 25mg/mL (Figure 2c). As shown in Figure 2d, the MTT absorbance increased with time, indicative of cell proliferation in the presence of 15mg/mL COS-OKA1- 3. This was similar to the results of the control treatment from days 1 to 6, signifying the in vitro biocompatibility of COS-O-KA1-3.
Zeta potential measurement
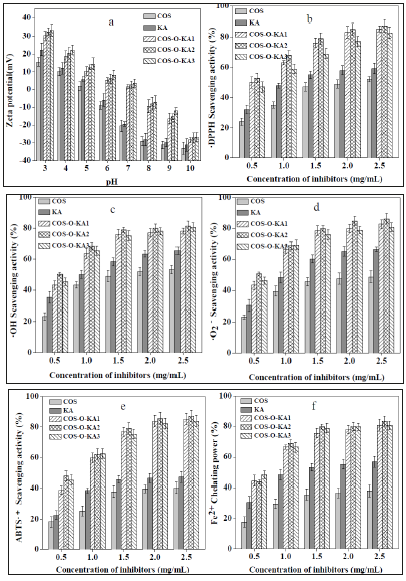
The surface charge of COS-O-KA1-3 was denoted by zeta potential (Figure 3a). At acidic pH, COS-O-KA became polycationic and more protonated amine groups were formed as the DS of the COS-O-KA reduced, resulting in a higher zeta potential [40]. It was confirmed that COS-O-KA3 with least DS of 0.98 displayed maximum charge density (33.33mV). At alkaline pH, the molecules were deprotonated with negative surface charge and were aggregated through intramolecular hydrogen bonding. Thus, DS altered the pKa of COS, protonating COS-O-KA1-3 differently from COS under identical pH conditions.
DPPH• scavenging activity
The DPPH• scavenging capacities of the samples increased in a concentration-dependent mode (Figure 3b). The DPPH• scavenging capacities of 2.5mg/mL of COS, KA, COS-O-KA1, COS-O-KA2, and COS-O-KA3 were 52.28%, 59.33%, 85.21%, 87.35%, and 82.83%, respectively. The relatively superior scavenging ability of COSO- KA1-3 could be ascribed to the existence of active amine and 5-hydroxypyranone groups in the structure. We have noticed that the order of scavenging activities of COS derivatives was as follows: COS-O-KA2 (DS = 1.22±0.4) > COS-O-KA1(DS = 1.41±0.3) >COS-OKA3 (0.98±0.5). This result indicated that COS derivatives, which has the medium DS was showed the strongest DPPH• scavenging activities. Hence, we assumed that when the γ-pyranone structure that contains an enolic hydroxyl group, active hydroxyl, and active amino of COS-O-KA get to the right ratio [41], the COS-O-KA could show the strongest DPPH• scavenging activity [42].
OH• scavenging activity
Figure 3c shows the COS, KA, and COS-O-KA1-3 scavenge •OH generated by the Fenton reaction. The •OH scavenging activity was increased obviously with the increased concentration of samples. The percentage scavenging effect of COS-O-KA1-3 ranged from 43.32-78.12%, 50.31-81.35%, 45.31-80.64%, respectively, which was higher than that of COS (23.30-53.28%) and KA (35.50- 65.53%), at concentrations of 0.5-2.5 mg/mL. It proved that COSO- KA1-3 had stronger •OH scavenging ability than COS and KA. The probable mechanisms of the scavenging activity of COS-O-KA1-3 against •OH may be as followings [5,15]: (1) The enolic hydroxyl groups of γ-pyranone structure and the hydroxyl groups of COS skeleton in the COS-O-KA polymer can react with •OH by the typical H-abstraction reaction. (2) The residual free amino groups (−NH2) of COS-O-KA1-3 polymer can react with •OH. (3) The protonated-NH2 groups (NH3+) can also react with •OH in solution through addition reaction (Figure 3).
Figure 3:a: Zeta potential of COS, KA, and COS-O-KA1-3(1mg/mL) in different pH. Scavenging activities on DPPH•(b), •OH(c), •O2−(d), ABTS•+(e), and Fe2+ (f) chelating power.
•O2− scavenging activity
The samples exhibited remarkable •O2− scavenging activity, which correlated significantly with the increase in the concentrations (P<0.05) (Figure 3d). At 2.5mg/mL, the scavenging abilities of COS-O-KA1-3 were above 80%. Nevertheless, the scavenging activity of COS and KA were much lower than COSO- KA1-3, with the scavenging activity of 48.74% and 66.68% at 2.5mg/mL, respectively. Based on the free-radical theory, the COSO- KA1, COS-O-KA2, and COS-O-KA3, which have alcohol or phenolic hydroxyl groups or amino groups in their molecular chain, can react with free radicals to yield stable macroradicals [5]. Thus, the active hydroxy and amine groups in COS-O-KA played main role in scavenging •O2− [24].
ABTS•+ scavenging activity
Our results revealed that the ABTS•+ scavenging activities of the samples shared similar trends that increased slowly with the increased concentration, and with the similar results in the DPPH• assay (Figure 3e). It is notable that the scavenging activities of COSO- KA1-3 against ABTS•+ were increased slowly (0.5-2.5mg/mL) and reached the plateau at 2.0mg/mL (83.87%, 85.76%, 82.33%, respectively), which was much greater than that of COS (39.65%), and KA (46.54%). Hence, we assume that the phenolic hydroxyl groups in the COS-O-KA polymer play a vital role in the hydrogenand electron-donating ability [38].
Assay of Fe2+ chelating activity
The Fe2+ chelating capacity is an indicator of antioxidant potency since the antioxidants are capable of hindering the formation of red Fe2+-ferrozine complex [2]. The results are summarized in Figure 3f. The Fe2+ chelating capacities of the samples were all concentration dependent. In particular, COS-O-KA1-3 displayed higher Fe2+ activity than COS and KA. The chelation efficiencies for 2.5mg/mL of COS, KA, COA-O-KA1, COA-O-KA2, and COA-O-KA3 were 37.85%, 56.79%, 80.88%, 83.52% and 80.65%, respectively. It was demonstrated earlier that the molecules that contain two or more of the -OH, -SH, -COOH, -PO3H2, C=O, -NR2, -S-, and -Ofunctionalities, generally display metal-chelating activities [43]. The structures of COS and KA include β-1,4-linked glucosamine (-OH, C=O, -NR2, and -O-) and 5-hydroxypyranone, respectively. Consequently, the chelating capacity of COS-O-KA was attributed to its distinctive catechol fragment and the numbers and positions of the functional groups. Grafting catechol group onto COS resulted in an enhanced Fe2+ chelating capacity and offered a route for the development of novel COS-based polymers for chelation [24,44].
Animal toxicity study
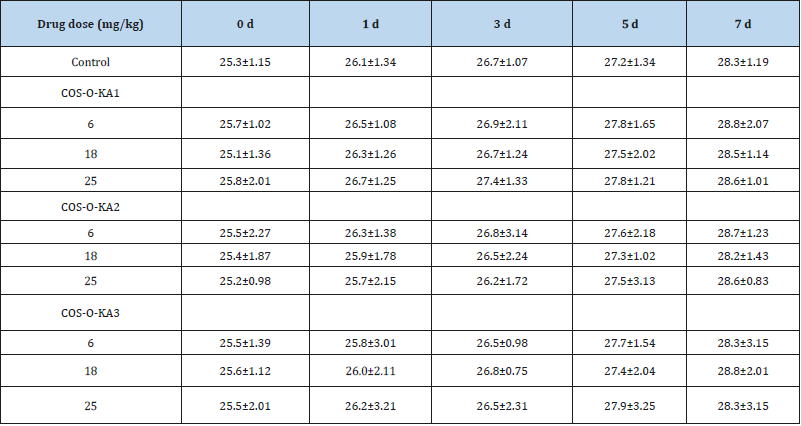
Animal toxicity studies of COS-O-KA1-3 were also tested conducted using healthy female SLACKM mice (6-8 weeks old and weighing 20.0±2.0 g) by tail vein injection at one control group and three groups for drug administration (6, 18, and 25mg/kg, respectively). After 7 days, the mice in the three drug administered groups did not develop any clinical signs of toxicity even at 25mg/ kg dose. The weight of each group increased gradually as did that of the control group (Table 2). Visual inspection of the animals did not reveal any illness and no mortality occurred. Therefore, the in vitro and in vivo studies established the non-/low-toxicity of COSO- KA1-3 (Table 2).
Table 2:Weight changes of mice in each group (X±S, n=10).
Conclusion
It was demonstrated that COS-O-KA polymer exhibited outstanding scavenging capacities against DPPH•, •OH, •O2−, ABTS•+, and Fe2+ chelating power, all of which were superior to those of native COS and KA. These are possibly due to the active hydroxy and amine groups in the COS-O-KA chains, which play a vital role in the free radical scavenging. Furthermore, the non-cytotoxic nature and biocompatibility of COS-O-KA afford opportunities for the future applications of this antioxidant material (Figure 1).
Author Contributions
Xiaoli Liu designed the study, collected test data, interpreted the results and drafted the manuscript. 273 Wancui Xie and Xihong Yang completed the statistical analysis of the data. Xiaobei Zhan and 274 Wenshui Xia participated in its design and revised the manuscript.
Acknowledgment
This work was granted financial support from National Natural Science Foundation of China (No. 279 31700709, 31671825), Natural Science Foundation of Jiangsu Province (No. BK20160180), National 280 First-Class Discipline Program of Food Science and Technology (JUFSTR20180201), and 281 Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
References
- Kwak SY, Yang JK, Choi HR, Park KC, Kim YB, et al. (2013) Synthesis and dual biological effects of hydroxycinnamoyl phenylalanyl/prolyl hydroxamic acid derivatives as tyrosinase inhibitor and antioxidant. Bioorganic & Medicinal Chemistry Letters 23(4): 1136-1142.
- Liu J, Luo J, Ye H, Sun Y, Lu Z, et al. (2010) In vitro and in vivo antioxidant activity of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydrate Polymers 82(4): 1278-1283.
- Liu J, Wen XY, Lu JF, Kan J, Jin CH (2014) Free radical mediated grafting of chitosan with caffeic and ferulic acids: Structures and antioxidant activity. Int J Biol Macromol 65: 97-106.
- Topal F, Nar M, Gocer H, Kalin P, Kocyigit UM, et al. (2016) Antioxidant activity of taxifolin: an activity-structure relationship. Journal of Enzyme Inhibition and Medicinal Chemistry 31(4): 674-683.
- Wan, Xu Q, Sun Y, Li H (2013) Antioxidant activity of high molecular weight chitosan and N, O-quaternized chitosans. J Agric Food Chem 61(28): 6921-6928.
- You J, Luo Y, Wu J (2014) Conjugation of ovotransferrin with catechin shows improved antioxidant activity. J Agric Food Chem 62(12): 2581-2587.
- Nazarzadeh Zare E, Mansour Lakouraj M, Mohseni M (2014) Biodegradable polypyrrole/dextrin conductive nanocomposite: synthesis, characterization, antioxidant and antibacterial activity. Synthetic Metals 187(C): 9-16.
- Saikia JP, Banerjee S, Konwar BK, Kumar A (2010) Biocompatible novel starch/polyaniline composites: characterization, anti-cytotoxicity and antioxidant activity. Colloids Surf B Biointerfaces 81(1): 158-164.
- Zare EN, Lakouraj MM, Mohseni M, Motahari A (2015) Multilayered electromagnetic bionanocomposite based on alginic acid: characterization and biological activities. Carbohydr Polym 130(C): 372-380.
- Córdoba LJP, Sobral PJ (2017) Physical and antioxidant properties of films based on gelatin, gelatin-chitosan or gelatin-sodium caseinate blends loaded with nanoemulsified active compounds. Journal of Food Engineering 213: 47-53.
- Wu GJ, Wu CH, Tsai GJ (2015) Chitooligo-saccharides from the shrimp chitosan hydrolysate induces differentiation of murine RAW264.7 macrophages into dendritic-like cells. Journal of Functional Foods 12: 70-79.
- Liu X, Xia W, Jiang Q, Yu P, Yue L (2018) Chitosan oligosaccharide-N-chlorokojic acid mannich base polymer as a potential antibacterial material. Carbohydr Polym 182: 225-234.
- Liu X, Xia W, Jiang Q, Xu Y, Yu P (2014) Synthesis, characterization, and antimicrobial activity of kojic acid grafted chitosan oligosaccharide. J Agric Food Chem 62(1): 297-303.
- Ma Z, Kim D, Adesogan AT, Ko S, Galvao K, et al. (2016) Chitosan Microparticles Exert Broad-Spectrum Antimicrobial Activity against Antibiotic-Resistant Micro-organisms without Increasing Resistance, ACS Applied Materials & Interfaces 8(17): 10700-10709.
- Xia W, Liu P, Zhang J, Chen J (2011) Biological activities of chitosan and chitooligosaccharides. Food Hydrocolloids 25(2): 170-179.
- Pasanphan W, Chirachanchai S (2008) Conjugation of gallic acid onto chitosan: an approach for green and water-based antioxidant. Carbohydrate Polymers 72(1): 169-177.
- Hou Z, Shankar YV, Liu Y, Ding F, Subramanion JL, et al. (2017) Nanoparticles of short cationic peptido polysaccharide self-assembled by hydrogen bonding with antibacterial effect against multidrug-resistant bacteria. ACS Appl Mater Interfaces 9(44): 38288-38303.
- Rui L, Xie M, Hu B, Zhou L, Saeeduddin M, et al. (2017) Enhanced solubility and antioxidant activity of chlorogenic acid-chitosan conjugates due to the conjugation of chitosan with chlorogenic acid. Carbohydr Polym 170: 206-216.
- Sun L, Sun J, Chen L, Niu P, Yang X, et al. (2017) Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr Polym 163:81-91.
- Kim S, Requejo KI, Nakamatsu J, Gonzales KN, Torres FG, et al. (2017) Modulating antioxidant activity and the controlled release capability of laccase mediated catechin grafting of chitosan. Process Biochemistry 59: 65-76.
- Wei L, Li Q, Tan W, Dong F, Luan F, et al. (2017) Synthesis, characterization, and the antioxidant activity of double quaternized chitosan derivatives. Molecules 22(3): 501.
- Liu M, Min L, Zhu C, Rao Z, Liu L, et al. (2017) Preparation, characterization and antioxidant activity of silk peptides grafted carboxymethyl chitosan. International Journal of Biological Macromolecules 104: 732-738.
- Bhattacharya, Misra BN (2004) Grafting: a versatile means to modify polymers: Techniques, factors and applications, Progress in Polymer Science 29(8): 767-814.
- Xie M, Hu B, Wang Y, Zeng X (2014) Grafting of gallic acid onto chitosan enhances antioxidant activities and alters rheological properties of the copolymer. Journal of Agricultural and Food Chemistry 62(37): 9128-9136.
- Bingi C, Emmadi NR, Chennapuram M, Poornachandra Y, Kumar CG, et al. (2015) One-pot catalyst free synthesis of novel kojic acid tagged 2-aryl/alkyl substituted-4H-chromenes and evaluation of their antimicrobial and anti-biofilm activities. Bioorganic & Medicinal Chemistry Letters 25(9): 1915-1919.
- Emami S, Ghafouri E, Faramarzi MA, Samadi, H Irannejad N, et al. (2013) Mannich bases of 7-piperazinylquinolones and kojic acid derivatives: Synthesis, in vitro antibacterial activity and in silico study. European Journal of Medicinal Chemistry 68 : 185-191.
- Hussein Ali SH, Arulselvan P, Hussein MZ, Fakurazi S, Saifullah B, et al. (2018) Evaluate the cytotoxicity of kojic acid nanocomposites on melanoma cells. Anticancer Agents Med Chem 36: 45-55.
- Halasa I, Reva L, Lapinski H, Rostkowska R, Fausto, et al. (2016) Conformers of kojic acid and their near-ir-induced conversions: long-range intramolecular vibrational energy transfer. J Phys Chem A 120(17): 2647-2656.
- Lakhawat S, Chaudhary J, Pathak AN (2014) Production of kojic acid by aerobic aspergillus fermentation. Bio Processing Journal 13(3): 62-69.
- M Saraei, G Zarrini, M Esmati, L Ahmadzadeh (2017) Novel functionalized monomers based on kojic acid: snythesis, characterization, polymerization and evalution of antimicrobial activity, Designed Monomers and Polymers 20(1) : 325-331.
- Chaudhary J, Lakhawat S, Pathak AN (2015) Elucidation on enhanced application of synthesised kojic acid immobilised magnetic and chitosan tri-polyphosphate nanoparticles as antibacterial agents. IET Nanobiotechnology 9(6): 375-380.
- Hussein Ali SH, Zowalaty ME, Hussein MZ, Ismail M, Dorniani D, et al. (2014) Novel kojic acid-polymer-based magnetic nanocomposites for medical applications. Int J Nanomedicine 9: 351-362.
- Uher M, Hudecová D, Brtko J, Dobias J, Kováč J, et al. (1989) Antifungal preparation. CS Patent 259592.
- Feng Y, Xia W (2011) Preparation, characterization and antibacterial activity of water-soluble O-fumaryl-chitosan. Carbohydrate Polymers 83(3): 1169-1173.
- Chung YC, Chen CY (2008) Antibacterial characteristics and activity of acid-soluble chitosan. Bioresour Technol 99(8): 2806-2814.
- Li P, Poon YF, Li W, Zhu WY, Yeap SH, et al. (2010) A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat Mater 10:149.
- Kouassi MC, Thébault P, Rihouey C, De E, Labat BA, et al. (2017) Carboxymethylpullulan grafted with aminoguaiacol: synthesis, characterization, and assessment of antibacterial and antioxidant properties. Biomacromolecules 18(10): 3238-3251.
- Hu Q, Wang T, Zhou M, Xue J, Luo Y (2016) In vitro antioxidant-activity evaluation of gallic-acid-grafted chitosan conjugate synthesized by free-radical-induced grafting method. J Agric Food Chem 64(29) :5893-5900.
- Liu X, Jiang Q, Xia W (2018) One-step procedure for enhancing the antibacterial and antioxidant properties of a polysaccharide polymer: kojic acid grafted onto chitosan. Int J Biol Macromol 113 :1125-1133.
- Chang SH, Lin H, Wu GJ, Tsai GJ (2015) pH Effects on solubility, zeta potential, and correlation between antibacterial activity and molecular weight of chitosan. Carbohydr Polym 134: 74-81.
- Hashemi SM, Emami S (2015) Kojic acid-derived tyrosinase inhibitors: synthesis and bioactivity. Pharmaceutical and Biomedical Research 1(1): 1-17.
- Ahn SM, Rho HS, Baek HS, Joo HS, Hong YD, et al. (2011) Inhibitory activity of novel kojic acid derivative containing trolox moiety on melanogenesis. Bioorg Med Chem Lett 21(24): 7466-7469.
- Yuan YV, Bone DE, Carrington MF (2005) Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro, Food Chemistry 91(3): 485-494.
- Brzonova, Steiner W, Zankel W, Nyanhongo GS, Guebitz GM (2011) Enzymatic synthesis of catechol and hydroxyl-carboxic acid functionalized chitosan microspheres for iron overload therapy. European Journal of Pharmaceutics and Biopharmaceutics 79(2): 294-303.
© 2019 Xiaoli Liu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






