- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
Surface Modifications of Carbon Fibers with Different Graphitization Indexes to Produce Cu/Carbon Fiber Composites by Electroless Process
Couto AB1, Matsushima JT1, Edwards ER2 and Ferreira NG1*
1 Instituto Nacional de Pesquisas Espaciais, Brazil
2 Departamento de Ciências Exatas e Tecnologia, Brazil
*Corresponding author: Ferreira NG, Instituto Nacional de Pesquisas Espaciais, Brazil
Submission: December 10, 2018;Published: December 14, 2018

ISSN 2578-0271 Volume4 Issue5
Abstract
Copper/carbon fiber (Cu/CF) composites by electroless Cu deposition were studied on carbon fibers (CF) produced at three different heat treatment temperatures (HTT) of 1000, 1500, and 2000 °C. The HTT control led to different CF graphitization indexes that influenced their chemistry surfaces. As a consequence, the electrochemical and chemical pre-treatments of the CF played distinct role by forming different oxygen functional groups also influencing the Cu deposition. CF substrates as well as Cu/CF composites were analyzed by Raman spectroscopy, Fourier transform infrared spectroscopy (FTIR), X-ray diffraction, and scanning electron microscopy. FTIR results revealed that both pre-treatments promoted CF surface modifications concerning the OH stretching vibrations for the three HTT studied. Nonetheless, the OH stretching vibration was more intense for all electrochemically pre-treated CFs. Moreover, the C-O and C=O stretching vibrations also appeared for the electrochemically pre-treated CF1000. Cu electroless deposition showed a strong dependence on the CF microstructure acquired after the HTT as well as on the pre-treatment step. The electrochemical pre-treatment was mandatory to obtain metallic Cu deposits on CF substrates. Regarding the nitrate electrochemical reduction, Cu/CF composites obtained from electrochemically treated CFs showed the nitrate reduction to nitrite followed by ammonia formation.
Keywords: Carbon fiber; Graphitization index; Copper deposition; Electroless process; Heat treatment temperature
Introduction
Deposition of metal particles on carbon fibers (CF) is important to produce composite that could be considered as a threedimensional material with large surface area for electrochemical applications. Copper (Cu) is the most common chemical element considering the transition metals since it presents excellent electrical and thermal conductivity. Particularly, Cu was adopted considering its use as potential electrode material to study the nitrogen compounds removal from the polluted waters [ref]. The control of nitrogen species is of great interest since the accumulation of these species on surface and ground water is linked to a high number of ecological and human health concerns [1]. In this sense, the development of methodologies economically viable and environmentally friendly has been studied for this purpose [2,3].
There are many attempts to produce Cu/CF composites by different routes like, liquid infiltration process [4], sputtering [5], electrodeposition [6], physical vapor deposition (PVD) [7], wetness technique [8], and electroless deposition [9]. The electroless plating has emerged in recent years due to its low cost, fast deposition rate, good filling capability, and low process temperatures [10]. Thus, the preparation of this type of composite using electroless process for application in electrochemical removal of nitrogen species in water is proposed in this paper.
CF substrates may be produced from different precursors and at different heat treatment temperature (HTT), which may promote different organization indexes on the CF structures [11]. In the Cu electroless process, the CF chemical inertness may result in a poor adhesion of the Cu. Thus, a hard pre-treatment is needed for cleaning the surface and creating anchor points on the CF samples [12]. These anchor points are groups that appear on the CF surface, such as, hydroxyl (O-H), carboxylic (C=O) and carbonylic (C-O), which have covalent bonds at the edge and at the structural defects on CF samples. The specific pre-treatments can be obtained using different oxidation techniques, such as, oxygen plasma [13] boiling in strong acids [14], ozone exposure [15] and electrochemical oxidation [16]. It is known that different oxygen-containing groups are produced using different oxidation techniques or exposures. Furthermore, the result of the oxidation process as a function of both the amount of oxygen as well as the type of carbon-oxygen groups may also depend on the nature of CF surface.
We present the Cu/CF composites formation by electroless process on CF produced at three different HTT of 1000, 1500, and 2000 °C. In this context, the goal of this work is to investigate the relationship between the HTT influence on CF structures as well as on their surface pre-treatments (chemical and electrochemical) on the Cu electroless deposition. In addition, these Cu/CF composites were used as electrode for electroreduction of nitrogen species.
Experimental
Materials
The CF samples were produced from polyacrylonitrile (PAN) precursor at different HTT of 1000, 1500 and 2000 °C using temperature steps of 330 °C h-1 under inert atmosphere of nitrogen, reaching the maximum during 30min up to its cooling down to room temperature. The CF samples were analyzed in the conditions of carbon fibers without pre-treatment (WT-CF), carbon fibers with chemical pre-treatment (CT-CF) and carbon fibers with electrochemical pre-treatment (ET-CF). The numbers that appear in the sequence of these notations are related to CF HTT.
Pre-treatment step on the CFs
Two surface pre-treatments were carried out on the CF samples: (1) for chemical process the CF were dispersed in boiling acetone for 5min and sonification with ultrasonic vibration using an ultrasonic probe of Sonic Model VCX 750W for 5min in a H2SO4/ K2Cr2O7 solution; (2) for electrochemical process, the samples were anodically polarized using 0.5mol L-1 H2SO4 in a fixed potential of 2.0V for 30min.
Cu electroless process
Prior to the Cu electroless process, the sensitization on the CF was achieved using a solution of 40mLL-1 HCl containing 0.04mol L-1 SnCl2 for 5min and the activation was made using a solution containing 7x10-4molL-1 PdCl2 with 2.5mL L-1 HCl for 5min. These steps are necessary for the subsequent reactions during the electroless plating. The electroless Cu deposition was carried out for 3min at room temperature and pH 12. The bath composition was 0.1mol L-1 CuSO4 + 0.2mol L-1 KNaC4H4O6 + 17.5mL L-1 HCHO + 0.5mol L-1 NaOH. The CF samples after the Cu electroless process was named as Cu/CF composites.
Morphological and structural analysis
The microstructure of both CF and Cu/CF composites was characterized by Raman and by FTIR-ATR analyses. Raman spectra were recorded by a Renishaw microscope system 2000 in backscattering configuration at room temperature, employing 514.5nm argon-ion laser. These measurements allowed verifying the material structural changes after the pre- treatments based in the relative intensity of D and G bands, (ID/IG). Fourier transform infrared (FTIR) spectrometer with attenuated total reflectance (ATR) Model Spectrum 100 from Parkin Elmer equipment was used to monitor the anchor points associated with the functional groups on CF surfaces promoted by these pre-treatments.
Cu/CF composite morphologies obtained by electroless process were analyzed by Scanning Electronic Microscopy (SEM) using a Jeol equipment JSM-5310. X-ray Diffraction (XRD) patterns were used in order to characterize the crystalline phases of the Cu deposits on CT-CF and ET-CF using a PAN analytical model X’Pert Powder diffractometer with the CuKα (λ = 1.54Å), set at 45kV and 25mA, in the ω/2θ configuration with ω = 1o and 2θ varying from 10 to 100o.
Nitrate electrochemical reduction
The electrochemical reduction of nitrate on the Cu/CF composites was evaluated using the linear sweep voltammetry (LSV), at 5mV s-1, in Britton-Robinson (BR) buffer solutions (pH=3.0) with and without the presence of 10-2mol L-1 KNO3. All the electrochemical measurements were made using Autolab PGSTAT 302 equipment with a three-electrode cell. The geometric area of the CF films in contact with the electrolyte was 1cm2. A platinum mesh served as a counter electrode and Ag/AgCl/KCl(sat) was used as the reference electrode.
Results and Discussions
Surface pre-treatment analyses
Raman spectroscopy analyses: HTT effect on the carbonization process for the PAN precursor conversion into CF was considered in this work, since a strong dependence on the CF microstructural properties with the HTT had been verified in our previous studies [11,17,18]. In those studies the first and second order Raman spectra of the CF produced at three different HTT were discussed. In this context, the Full Width at Half Maximum (FWHM) values and the relative intensity (ID/IG) between the D and G bands were used to evaluate the disorder degree of graphitic materials [19,20]. Almeida et al. [17] discussed that these parameters are important to evaluate the CF graphitization index associated to the HTT increase, which promotes a high organization of the graphitic layers. Thus, in this work, this set of experimental parameters calculated for the CF produced at three different HTT were used as a reference to analyse the change on the CF microstructure after the chemical and electrochemical pre-treatments. This analysis, associated to CF graphitization indexes, was determinant in the CF pre-treatment step, because according to literature the adsorption capacity on the deposition process is influenced by the CF chemical surface [21,22]. Therefore, these results helped to establish specific conditions for the electroless Cu deposition onto CF. Figure 1 shows the Raman spectra obtained for CF samples treated at 2000 °C, after chemical (CT-CF2000), electrochemical (ET-CF2000), and without (WT-CF2000) pre-treatments. The spectra also were analyzed for the CF1000 and CF1500 after the chemical and electrochemical pre-treatment (not shown). In their cases, the CF microstructural properties did not show significant changes. This behavior may be associated to the low structural organization for CF1000 and CF1500 samples.
For the first order spectrum for CF2000, the D band located at 1355-1360cm-1 is a good indication regarding the crystalline structure disorder while the G band located at 1570-1600cm-1, is a characteristic of the ordered graphitic structure. At approximately 1620cm-1 and 2700cm-1 the D’ and G’ bands are found for the CF produced at HTT higher than 1500 °C. It is important to point out that the WT-CF2000 has been characterized as graphite-like material due to the increase in the HTT as reported in previous work [11,17,18]. According to Almeida et al. [17], the CF treated at temperatures between 1500-2000 °C may lose most of its noncarbon impurities leading to a graphite like structure. On the other hand, for samples treated up to 1000 °C, the noncarbon elements are removed as volatiles, such as H2O, HCN, NH3, CO, CO2, N2, etc [11] and the PAN precursor loses about 50% of its original mass when converted to CF that contains only carbon and some nitrogen (6%). Table 1 presents the FWHM and ID/IG values obtained from the first order spectra shown in Figure 1.
Table 1:Parameters obtained from the fitting of the Raman spectrum for: carbon fiber without chemical treatment (WTCF), carbon fibers with chemical treatment (CT-CF) and carbon fibers with electrochemical treatment (ET-CF) treated at the temperature of 2000 °C.
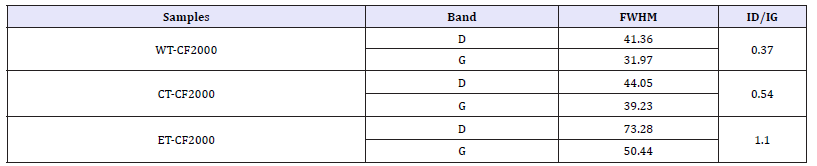
Figure 1:Raman spectra at 514.5nm for: carbon fiber without chemical treatment (WT-CF), carbon fiber with chemical treatment (CTCF) and carbon fiber with electrochemical treatment (ET-CF) treated at the temperature of 2000 °C.

A considerable change into FWHM and ID/IG values is verified for the three samples. The higher FWHM and ID/IG values for the ET-CF2000 revealed an enlargement of D and G bands compared to those for the WT-CF2000. These parameters show the highest degree of structure disorder for the ET-CF2000 due to its hard oxidation process. Koslowski & Sherwood [23,24] also discussed the effectiveness of the electrochemical oxidation on the graphitic structure disorder, where the graphitic C1s peak from XPS results loses its asymmetric tail at higher potential due to the exfoliation of the CF surface [25].
FTIR-ATR spectroscopy analyses: FTIR-ATR analyses were carried out to evaluate the chemical surface without pre-treatment and after chemical as well as electrochemical pre-treatments regarding the presence of functional groups on the CF surfaces for the three HTT studied. Figure 2 shows the graphs of the FTIRATR analyses of the carbon fibers: (A) without pre-treatment (WT-CF) (B) with chemical pre-treatment (CT-CF) and (C) with electrochemical pre-treatment (ET-CF) wherein all of them were thermally treated at temperatures of 1000, 1500 and 2000 °C, respectively. For a better visualization of the spectra changes, the graphs were divided in regions concerning each functional group: (i) the band between 2500-3600cm-1 corresponding to the functional group (OH), (ii) the band between 1670-1760cm- 1 corresponding to the functional group (C=O) and (iii) the band between 1000-1260cm-1 corresponding to the functional group (CO), which were responsible for changing the surface conditions of the CF as mentioned by other authors [12-14].
Figure 2:FTIR-ATR results with peaks identified for: (A) Carbon fiber without chemical treatment (WT-CF) treated at the temperature of 1000, 1500 and 2000 °C; (B) Carbon fibers with chemical treatment (CT-CF) treated at the temperature of 1000, 1500 and 2000 °C; (C) Carbon fibers with electrochemical treatment (ET-CF) treated at the temperature of 1000, 1500 and 2000 °C.

By analyzing the FTIR-ATR spectra for all WT-CF, a notable difference was verified for WT-CF1000 due to the OH stretching vibration of the carboxylic groups that occurred at ~2900cm- 1. The presence of these functional groups may be associated to the oxidative state resulting from the graphitic structure disorder which was verified for CF1000. After the chemical and electrochemical pre-treatments, the OH stretching vibration of the carboxylic groups begins to intensify leading to the appearance of a more intense FTIR band at ~2900cm-1. By comparing the FTIR-ATR spectra of the CT-CF, slight surface modifications can be observed in the Figure 2(B) compared to those of Figure 2(A). The OH stretching vibrations of the carboxylic groups were observed for these three samples. Nonetheless, for CT-CF1000 this vibration was more intense. On the other hand, a strong surface modification was observed for ET-CF samples, by comparing Figure 2(C) to those of Figure 2(A) related to the OH stretching vibration. One interesting aspect was observed for ET-CF1000 associated to C-O and C=O stretching vibrations as shown in Figure 2(C). The presence of these vibrations on this sample may be related to its more favorable oxidation after the electrochemical pre-treatment due to its lowest structural organization.
Cu/CF composite analyses
SEM analyses: The top view SEM images of the electroless Cu deposits on CT-CF and ET-CF are depicted in Figure 3. Thinner Cu deposits with particles uniformly distributed covering the entire CT-CF surface were observed. These deposits were very homogeneous, without the cracks or delaminations, regardless of the HTT. This homogeneity may be justified by similar surface chemical properties related to the presence of the C-O, C=O and OH groups evidenced in FTIR spectrum (Figure 2), since the copper adsorption of Cu2+ ions occurs through electrostatic force between Cu2+ ions and these oxygen-containing groups. On the other hand, the morphology of the Cu deposits on the ET-CF was influenced by the surface functionalization. The Cu deposit on the ET-CF1000 (Figure 3B1) seems to be thicker and it showed cracks. This behavior may be associated to an intense presence of C=O functional groups where it has the highest electrostatic interaction with Cu2+ in the reduction process [6], thus enhancing the Cu deposition. In addition, for samples ET-CF1500 (Figure 3B2) and ET-CF2000 (Figure 3B3), clusters above the Cu film layer also appeared. This characteristic may be related to a more effective electrochemical pre-treatment on the CF surface, which probably favored this deposition, as shown in FTIR analysis by the better definition of OH vibration in such pretreatment for all HTT.
Figure 3:SEM micrographs of the composite for: carbon fibers with chemical treatment (CT-CF) treated at the temperature of (A1)1000, (A2)1500 and (A3)2000 °C; carbon fibers with electrochemical treatment (ET-CF) treated at the temperature of (B1)1000, (B2)1500 and (B3)2000 °C.

XRD analyses: Figure 4 presents XRD patterns of the Cu deposits on CT-CF and ET-CF samples at HTT of 1000, 1500 and 2000 °C. According to the graphs, the deposited Cu phases were influenced by the microstructural difference of the CF, due to the different HTT, as well as by the pre-treatments on these substrates.
Figure 4(A) showed that the Cu electroless on the CT-CF led to the formation of three different copper phases (Cu metallic, CuO cubic, CuO monoclinic) including the presence of graphite phase from the CF substrate. On the other hand, for the electrochemically pre-treated CF (ET-CF), Figure 4(B), the appearance of two peaks related to Cu and Cu2O was verified. For ET-CF1000 sample only metallic Cu phase was observed whereas a mixture of phases containing cuprous oxide and metallic copper was identified in the ET-CF1500 and ET-CF2000 samples. As presented in the FTIR results (Figure 2C), the ET-CF1000 acquired well evident C=O groups, which probably favored the deposition of the metallic Cu phase. This process may be associated to the more effective presence of these functional groups that improved the reduction capacities of Cu2+ ions on ET-CF1000 surface. We speculate that the formation of these functional groups from anodic electrochemical pre-treatment is related to the presence of impurities and structural disorder on CF1000. It is noteworthy that the chemical pre-treatment did not favor the C=O groups formation as it can be seen in Figure 2(B). These results indicate the preferential metallic Cu deposition on anchoring points related to the C=O groups. They are also consistent with those reported by Li et al. [6]. According to them, the C=O groups present on their CF surface treated at 400 °C was responsible for the Cu reduction reaction. In addition, the cuprous oxide phase presence, specifically observed on the ETCF1500 and ET-CF2000, may be attributed to specific adsorption of copper through electrostatic force between Cu2+ ions and OH groups on the sample surfaces, as discussed in FTIR analyses (Figure 2(C)).
Figure 4:XRD patterns for electroless Cu deposits obtained for: (A) carbon fibers with chemical treatment (CT-CF) treated at the temperature of 1000, 1500 and 2000 °C; (B) carbon fibers with electrochemical treatment (ET-CF) treated at the temperature of 1000, 1500 and 2000 °C . The deposited Cu phases were identified using X’Pert High Score report of the PAN alytical.
Nitrate electrochemical reduction
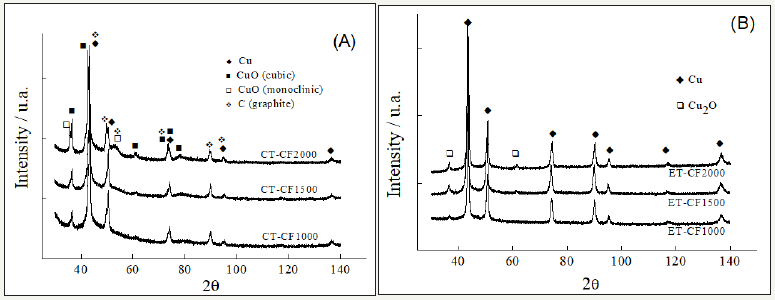
The electrochemical reduction of nitrate on the Cu/CF composites was tested in order to evaluate its effectiveness regarding the electrochemical removal of nitrogen species in water. Figure 5 depicts the LSV obtained in 10-3mol L-1 BR buffer solutions (pH = 3.0) with and without the 10-2mol L-1 KNO3 addition for all Cu/CF composites studied, considering both pre-treatments as well as at the three CF HTT. It is important to point out that the electrochemical behavior in the absence of nitrate was similar for all Cu/CF composites. Therefore, the LSV curves were not shown for Cu/CF1500 and Cu/CF2000 samples in Figure 5.
Figure 5:LSV recorded in BR acidic buffer solution for carbon fibers with chemical treatment (CT-CF) treated at the temperature of 1000, 1500 and 2000 °C and carbon fibers with electrochemical treatment (ET-CF) treated at the temperature of 1000, 1500 and 2000 °C.
By analyzing the LSV curves, some differences are evident. For ET-Cu/CF composites, the nitrate reduction gave rise to two regions of cathodic current, where a current shoulder located in C1 region is associated with the nitrate reduction to nitrite, while a current shoulder in C2 region is due to the nitrate conversion to ammonia. With few exceptions, this process has already been evidenced either in acidic or in strongly alkaline solutions on copper cathodes [26-30]. In those conditions, the nitrate reduction occurred by nitrite formation up to ammonia. Particularly, this process was more pronounced for the ET-CF1000 because of the pure metallic
Cu phase presence observed in this composite (Figure 3B). Just one well defined region of cathodic current at around -0.6 V (C1 region) is characterized for all CT-CF samples when compared to those for all ET/CF. This behavior is associated with the nitrate reduction to nitrite, probably due to the presence of copper oxide phases as previously reported in XRD analyses.Conclusion
The preparation of Cu/CF composites using the electroless process on CF, produced at different HTT, was influenced by the microstructural aspect as well as by the pre-treatments carried out on the substrates. CF treated at 1000 °C showed the lowest graphitic structural organization. This characteristic was crucial to modify its surface energy condition and, consequently, had an important role in the electrochemical and chemical pre-treatments by forming different surface oxygen functional groups. From FTIR analyses, the signal related to OH stretching vibration was evidenced after the chemical treatment for CF1500 and CF2000. On the other hand, the C-O and C=O stretching vibrations for CF1000 on electrochemically pre-treated were observed due to its oxidative state resulting from the graphitic structure disorder. XRD results, concerning the Cu phases formation, were clearly influenced by the different pretreatments on CF surface. Cu deposits on CF electrochemically pre-treated contain mainly the metallic Cu phase, attributed to an effective formation of C=O groups on CF surface. However, for the chemically pre-treated CF, three different phases of copper (Cu metallic, CuO cubic, CuO monoclinic) were obtained. Regarding the nitrate electroreduction, two processes were observed in the Cu/CF composites. The C1 process related to nitrite formation was more marked for Cu/CF composites produced after chemical pretreatment. Conversely, the occurrence of the nitrate reduction into nitrite followed by the ammonia (C2 process) may be related to the presence of the pure metallic Cu phase observed in the Cu/CF composite produced on the electrochemically pre-treated CF.
Acknowledgment
This paper is a contribution of the Brazilian National Institute of Science and Technology (INCT) for Climate Change funded by CNPq Grant Number 573797/2008-0 and FAPESP Grant Number 2016/13393-9. We also thank CAPES Grant Number 02491/09- 5 for the financial support and M.L. Brison and J.P.B. Machado for MEV and XRD analyzes, respectively.
References
- Gelberg KH, Church L, Casey G, London M, Roerig DS, et al. (1999) Nitrate levels in drinking water in rural New York State. Environ Res 80(1): 34- 40.
- Archna, Surinder KS, Ranbir CS (2012) Nitrate removal from ground water: a review. E-Journal of Chemistry 9(4): 1667-1675.
- Inam-Ul-Haque, Muqaddas T (2010) Electrochemical reduction of nitrate: a review. J Chem Soc Pak 32: 396-418.
- Prakasan K, Palaniappan S, Seshan S (1997) Thermal expansion characteristics of cast Cu based metal matrix composites. Composites 28(12): 1019-1022.
- Neubauer E, Korb G, Eisenmenger-Sittner C, Bangert H, Chotikaprakhan S, et al. (2003) The influence of mechanical adhesion of copper coatings on carbon surfaces on the interfacial thermal contact resistance. Thin Solid Films 433(1-2): 160-165.
- Wei L, Lei L, Cheng Z, Bin S, Wenbin H (2011) Effect of carbon fiber surface treatment on Cu electrodeposition: The electrochemical behavior and the morphology of Cu deposits. Journal of Alloys and Compounds 509(8): 3532-3536.
- Schrank C, Eisenmenger-Sittner C, Neubauer E, Bangert H, Bergauer A (2004) Solid State de-wetting observed for vapor deposited copper films on carbon substrates. Thin Solid Films 459(1-2): 276-281.
- Ma J, Park C, Rodriguez NM, Baker RTK (2001) Characteristics of copper particles supported on various types of graphite nanofibers. J Phys Chem B 105(48): 11994-12002.
- Byeon JH, Kim JW (2010) Novel electroless copper deposition on carbon fibers with environmentally friendly processes. J Colloid Interface Sci 348(2): 649-653.
- Tamayo-Ariztondo J, Córdoba JM, Odén M, Molina-Aldareguia JM, Elizalde MR (2010) Effect of heat treatment of carbon nanofibers on electroless copper deposition. Composites Science and Technology 70(16): 2269- 2275.
- Medeiros LI, Couto AB, Matsushima JT, Baldan MR, Ferreira NG(2012) Nanocrystalline diamond coating on carbon fibers produced at different temperatures: Morphological, structural and electrochemical study. Thin Solid Films 520(16): 5277-5283.
- Wang F, Arai S, Endo M (2004) Metallization of multi-walled carbon nanotubes with copper by electroless deposition process. Electrochem Commun 6: 1042-1044.
- Perakslis ED, Gardner SD, Pittman CU (1997) Surface composition of carbon fibers subjected to oxidation in nitric acid followed by oxygen plasma. J Adhes Sci technol 11(4): 531-551.
- Córdoba JM, Odén M (2009) Growth and characterization of electroless deposited Cu films on carbon nanofibers. Surface & Coatings Technology 203(22): 3459-3464.
- Fu X, Lu W, Chung DDL (1998) Ozone treatment of carbon fiber for reinforcing cement. Carbon 36(9): 1337-1345.
- Lindsay B, Abel ML, Watts JF (2007) A study of electrochemically treated PAN based carbon fibres by IGC and XPS. Carbon 45(12): 2433-2444.
- Almeida EC, Trava-Airoldi VJ, Baldan MR, Ferreira NG (2007) Correlation between chemical vapor deposited diamond and carbon fibers substrates. J Mat Sci 42(7): 2250-2254.
- Almeida DAL, Antunes EF, da Silva VQ, Baldan MR, Ferreira NG (2013) Growth of vertically aligned carbon nanotubes on carbon fiber: thermal and electrochemical treatments. J Solid State Electrochem 17(7): 1977- 1984.
- Ferrari AC, Robertson J (2000) Interpretation of raman spectra of disordered and amorphous carbon. Phys Rev B 61: 14095-14107.
- Ferrari AC, Robertson J (2001) Resonant raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys Rev B 64: 075414-1-075414-13.
- Tang YP, Liu L, Hu WB (2005) Loose porous composites of Ni/short carbon fibers prepared by electrodeposition. J Mater Sci 40(16): 4399- 4401.
- Chen S, Zeng H (2003) Improvement of the reduction capacity of activated carbon fiber. Carbon 41(6): 1265-1271.
- Koslowski C, Sherwood PMA (1985) X-Ray photoelectron-spectroscopic studies of carbon-fibre surfaces. J Chem Soc Faraday Trans 1 81: 2745- 2756.
- Koslowski C, Sherwood PMA (1986) X-ray photoelectron spectroscopic studies of carbon fibre surfaces vii-electrochemical treatment in ammonium salt electrolytes. Carbon 24: 357-363.
- Almeida EC, Diniz AV, Trava-Airoldi VJ, Ferreira NG (2005) Electrochemical characterization of doped diamond-coated carbon fibers at different boron concentrations. Thin Solid Films 485(1-2): 241- 246.
- Davis J, Moorcroft MJ, Wilkins SJ, Compton RG, Cardosi MF (2000) Electrochemical detection of nitrate at a copper modified electrode under the influence of ultrasound. Electroanalysis 12(17): 1363-1367.
- Reyter D, Bélanger D, Roué L (2008) Study of the electroreduction of nitrate on copper in alkaline solution. Electrochim Acta 53(20): 5977- 5984.
- Bouzek K, Paidar M, Sadilkova A, Bergmann H (2001) Electrochemical reduction of nitrate in weakly alkaline solutions. J Appl Electrochem 31(11): 1185-1193.
- Soropogui K, Sigaud M, Vittori O (2006) Alert electrodes for continuous monitoring of nitrate ions in natural water. Electroanalysis 18(23): 2354-2360.
- Badea BE (2009) Electrocatalytic reduction of nitrate on copper electrode in alkaline solution. Electrochim Acta 54(3): 996-1001.
© 2018 Ferreira NG . This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






