- Submissions

Full Text
Trends in Textile Engineering & Fashion Technology
Flexible Conductive Films Fabricated Using Exfoliated Graphite and Nanocellulose
Colman MME1*, Canesqui MA2, Tasic L3, Bartoli JR1 and Moshkalev S2
1 Faculty of Chemical Engineering, State University of Campinas, Brazil
2 Semiconductor and Nanotechnology Components Center, State University of Campinas, Brazil
3 Department of Organic Chemistry, State University of Campinas, Brazil
*Corresponding author: Colman MME, Faculty of Chemical Engineering, State University of Campinas, Campinas, Brazil
Submission: August 02, 2018;Published: August 07, 2018

ISSN 2578-0271 Volume4 Issue1
Abstract
Development of nanostructured composite materials based on exfoliated graphite and nanocellulose for applications in flexible electronics including smart textiles is described. High electrical conductivities, near 140kS/m, comparable with conventional conductive materials like metals, as well as good mechanical properties, were measured for the graphite-cellulose compound.
Introduction
Flexible highly conductive thin film materials have been gaining growing attention due to numerous possible applications in flexible electronics including for smart textiles in particular for local heating and sensing [1]. Graphene is a new promising highly conductive and flexible material that has been the target of numerous theoretical and experimental researches, due to the excellent physical and chemical properties [2] and a great potential in many fields of application such as composite materials, electronic devices, energy storage, batteries, capacitors and sensors. However, a limiting factor is still the lack of effective methods for mass production of graphene [3]. Here we present one approach developed to overcome this limitation, based on low cost processing method for exfoliation of graphite to produce a few and multilayer flexible graphene sheets that could be used in an industrial scale. It is known that the well-known graphite exfoliation method based on a chemical mechanism, introduces numerous defects in its structure, which could compromise its mechanical and electrical performance [4]. On the other hand, mechanical properties of films based solely on exfoliated graphene are not good enough for applications like in smart textiles when repetitive stretching and bending are applied. Thus graphene-based composite materials including high aspect (length/diameter) ratio reinforcing elements could be a choice for this particular application. Cellulose is a low-cost, abundant, biocompatible biopolymer whose modification is easy and ecofriendly. Due to interesting mechanical properties and high aspect ratio this material can also be used as reinforcements for adhesives, components of electronics devices, textiles, among others [5]. However, it is a hydrophobic material, so it does not dissolve in water or hydrophilic solvents. Medronho et al. [6] emphasized that cellulose is soluble in solvents with intermediate properties such as N-methylmorpholine N-oxide and ionic liquids. In this way, cellulose can act as an exfoliation agent on the surface of graphite, stabilizing its aqueous dispersions [3]. Summarizing, exfoliated graphite emerged as an excellent component to obtain new conductive nanostructured materials and cellulose, in turn, has an excellent potential for less aggressive eco-friendly graphite exfoliation and preparation of flexible conductive films for numerous applications in smart textiles.
Methodology
The preparation of the graphite exfoliation was performed according to Ferreira et al. [3] and divided into two stages. Two types of graphite were used: non-expanded and expanded, provided by the Nacional de Grafite Ltda (Brazil). Three different types samples were prepared: (a) 1NC, graphite non-expanded; (b) 2NC, graphite expanded and (c) 3NC, graphite expanded with acrylic emulsion binder. The first step fabrication consisted of the addition of the cellulose obtained by Mariño et al. [7] in 7% aqueous sodium hydroxide solution (NaOH, Sigma-Aldrich) and sonicated by a 7mm diameter probe at 70% amplitude and 90W (Hielscher, Model UP400ST) with pulses of 2s, in an ice bath. This graphite-cellulose mixture remained in the freezer for 1 hour at -6 °C. The second step was to prepare the graphite in 7% aqueous NaOH solution. Subsequently, the two phases were mixed and shaken in a water bath (Dubnoff) with mechanical stirrer (Marconi) at 133rpm for 18h. Another expanded graphite-cellulose compound containing 15% acrylic polymeric binder (Printofix CA, Archroma) was prepared in relation to the expanded graphite - cellulose mass. A homogenizer (Ultra Turrax) was used to mix the solution of this sample (3 NC).
The graphite-cellulose thin films were obtained by vacuum filtration of solutions using qualitative paper filters (Calgon), 7cm in diameter, after the stirring step. The samples were then kept for 24h at room temperature for drying and then compacted by calendering at 120 °C and opening between calender cylinders from 0.35 to 0.45mm. Nanostructured composites of graphite and cellulose nanosheets were obtained for electrical and flexible conductive films. The thicknesses of the so prepared films were measured with Mitutoyo micrometer. Electrical characterization of films was performed using the van der Pauw four-probed method (1959) with Agilent Instruments, B2912A Series Low Noise Power Source equipment. Morphological analyzes of the films were made via Scanning Microscopy, SEM (Hitachi, S-3400N).
Results and Discussion
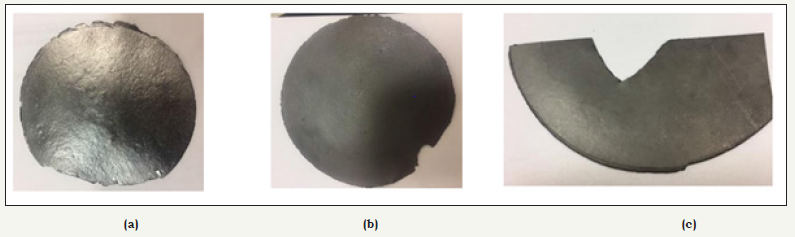
Figure 1 shows the films produced by vacuum filtration of the samples 1NC, 2NC and 3NC with the graphite non-expanded, expanded and acrylic emulsion binder expanded, respectively. The samples 1NC and 2NC presented improved mechanical properties due to addition of cellulose; however, the 3NC sample with higher content of graphite and polymeric binder showed no mechanical stability [8]. Then, to recompose the mechanical integrity of the graphite with the nanocellulose, 15% acrylic emulsion binder was added in relation to the mass of graphite and nanocellulose, and then added in 200mL of 7% NaOH aqueous solution. It was homogenized in Ultra Turrax for 20 minutes and 6000rpm, after this process the film was prepared (Figure 1C).
figure 1:Conductive nanosheets: 1NC, graphite non-expanded (a); 2NC, graphite expanded (b) and 3NC, graphite expanded with acrylic emulsion binder (c).
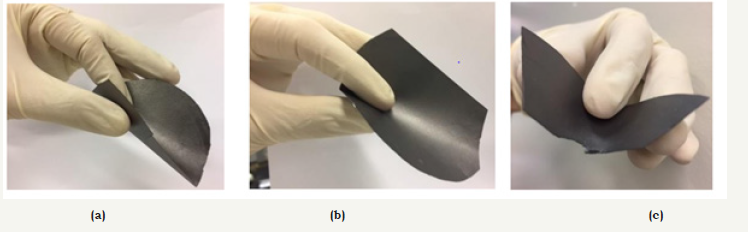
The conductivities of the samples were measured before and after calendaring that resulted in strong reduction of the resistivity (increase of the conductivity). For the samples 1NC and 2NC the best conductivities are typical for conductive materials, being near 140kS/m (resistivity of 0.7x10-4Ohm.cm) and 10kS/m (1.0x10- 2Ohm.cm), for the thicknesses of 10-2 and 8x10-2mm, respectively. Lower values the higher values of the 3NC sample resistivities (conductivity 5kS/m to 0.2mm) are due in part to a certain porosity observed in the material, in addition to the use of the polymeric dielectric binder. After calendaring, the best resistivities were measured for sample 1NC, being near, while higher values were obtained for samples 2NC and 3NC, being and 2x10-2Ohm.cm, respectively. It was noticed that the malleability of the samples was favored by adding of cellulose, as can be seen in Figure 2.
figure 2:Conductive films: 1NC, graphite NF-C type (a); 2NC, graphite expanded (b) and 3NC, graphite expanded with acrylic emulsion binder (c).
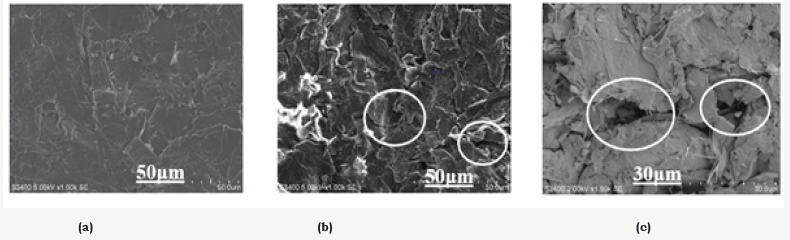
The micrographs (SEM, Figure 3) show random orientation of the graphite sheets within the film. However, the 1NC sample, with non-expanded graphite, showed a better uniformity and compact morphology, after the calendering process, than the 2NC and 3NC samples. The latter, with expanded graphite, showed a certain porosity in the surface of the samples, justifying lower conductivity in this material. In the 3NC sample with acrylic emulsion binder (Figure 3C) it is possible to observe the cellulose inclusions on the surface of the composite.
figure 3:SEM images of samples: 1NC, 2NC, and 3NC with acrylic emulsion binder and cellulose.
Conclusion
The use of cellulose was favorable for imporvement of mechanical properties (malleability) of nanographite flexible fims, both for expanded graphite or non-expanded graphite. However, the larger amount of expanded graphite relative to cellulose led to a more fragile material, even adding an acrylic emulsion binder. High electrical conductivities were found in the cellulose and non-expanded graphite composites after calendering process that reduced the thickness and improved the contact between the graphite nanofoils, with more uniform morphology seen by SEM microscopy. The best results in electrical conductivity obtained here, ~140 140kS/m, are comparable with those for conventional conductive materials like metals. High electrical conductivity and good mechanical properties of the present graphite-cellulose composite material open way to applications in smart textiles in particular for local heating, mechanical sensing and RFID.
References
- Liu Y, Sun B, Li J, Cheng D, An X, et al. (2018) Aqueous dispersion of carbon fibers and expanded graphite stabilized from the addition of cellulose nanocrystals to produce highly conductive cellulose composites.ACS Sustanaible Chem Eng 6(3): 3291-3298.
- Du W, Jiang X, Zhub L (2013) From graphite to graphene: direct liquidphase exfoliation of graphite to produce single- and fewlayered pristine graphene. J Mater Chem A 1(36): 10592-10606.
- Ferreira ES, Da Silva DS, Burgo TAL, Batista BC, Galembeck F (2017) Graphite exfoliation in cellulose solutions. Nanoscale 9(29): 10219- 10226.
- Stankovich S, Dikin DA, Piner RD, Kohlhaas KAA, Kleinhammes A, et al. (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45(7): 1558-1565.
- Morais JPS, De FRM, Moreirade SFM, Nascimento LD, Do NDM, et al. (2013) Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydrate Polymers 91(1): 229-235.
- Medronho B, Romano A, Miguel MG, Stigsson L, Lindman B (2012) Rationalizing cellulose (in)solubility: reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose 19(3): 581- 587.
- Mariño M, Lopez DSL, Duran N, Tasic L (2015) Enhanced materials from nature: nanocellulose from citrus waste. Molecules 20(4): 5908-5923.
- Van-Der PLJ (1959) A method of measuring the resistivity and hall coefficient on lamellae of arbitrary shape. Philips Technical Review 20: 220-224.
© 2018 Uwe Reischl . This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






