- Submissions

Full Text
Techniques in Neurosurgery & Neurology
Guinea Pig’s Spine as a Biomechanical Model in Neurology and Orthopedic
Marko Jumake Mitrović*, Anastasija Todorović and Mirjana Lazarević Macanović
Department of Radiology and Radiation Hygiene, University of Belgrade, Serbia
*Corresponding author:Marko Jumake Mitrović, Department of Radiology and Radiation Hygiene, Faculty of Veterinary Medicine, University of Belgrade, Bulevar oslobođenja, Belgrade, Serbia
Submission: May 25, 2023;Published: June 23, 2023
ISSN 2637-7748
Volume5 Issue3
Abstract
Animal models play an important role in the investigation of spine biomechanics. Guinea pigs are used in some neurological and orthopedic biomedical research, but the biomechanics of their spinal column is little discussed in the available literature. Therefore, the goal of this study was to point out the similarities and differences in the spinal column of humans and these animals, which can be significant when planning experiments.
Keywords:Biomechanics; Guinea pig; Spine
Introduction
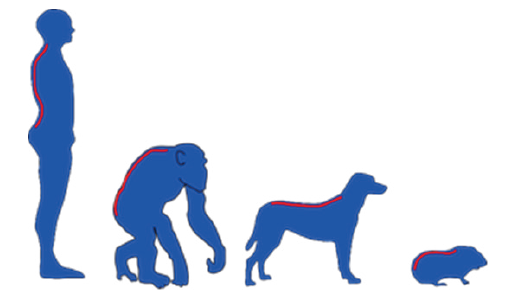
Guinea pigs (Cavia porcellus) are a herbivorous species of rodents from the family Caviidae which due to their low body weight, short reproductive cycle, calm temperament, as well as anatomical and physiological characteristics, are increasingly used as models in various studies [1]. In neurology and orthopedics, guinea pigs have been used to examine ischemic lesions in the spinal cord [2] and for the study of spontaneous and induced osteoarthritis [3]. It should be borne in mind that there are significant differences in the morphologic of the spinal columns of bipedal and quadruped organisms (Figure 1), and therefore in biomechanics, which can significantly impair the quality and applicability of the results obtained.
Figure 1:Presentation of the morphology of the spinal column in bipedal and
quadruped organisms.
In the available literature, there are various findings of different morphometric and/or biomechanics examinations of the spinal column performed on rabbits [4], dogs [5], pigs [6], sheep [7] and cattle [8,9]; however, the search for an optimal animal model is still ongoing. When using an animal model in tests on the spine, one should know the basic anatomical characteristics of the spinal column of a given animal, such as anatomical characteristics of the spinal column (number and morphology of vertebrae), as well as the direction of forces through the spine column.
Characteristics of the spine of guinea pigs
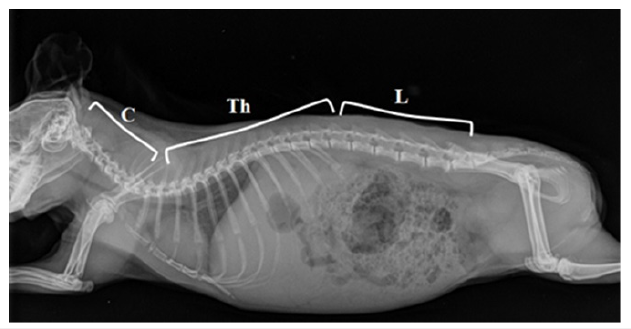
The spinal column of rodents has a total of 26 presacral vertebrae, composed of 7 cervical and 19 thoracolumbar vertebrae [10]. The cervical spine region of mammals is characterized by the smallest variations in the number of vertebrae, which is usually attributed to the pleiotropic function of Hox genes. In addition, variations in the number of cervical vertebrae are associated with an increased risk of prenatal mortality and neonatal cancer [10,11]. On the other side, the thoracolumbar region of the spine of guinea pigs shows greater variability, and in most individuals, it is composed of 13 thoracic and 6 lumbar vertebrae (Figure 2) [12].
Figure 2:X-ray image of a guinea pig in the later-lateral projection: Cervical (C), Thoracal (Th) and Lumbar
Vertebrae (L).
Is axial loading of the vertebral column present only in bipedal organisms?
A common misconception is that quadrupedal spines are not subjected to axial load like the upright, bipedal human spine is. In reality, however, it has been demonstrated that quadruped spines not only experience axial loads but also that they may in fact be higher than in humans. Indeed, high muscle and ligament forces act on the quadrupedal spine to constrain its movement in the frontal and sagittal planes [13]. It is important to note that the trabeculae of the vertebral bodies of both bipeds and quadrupeds are oriented from endplate to endplate, implying that they undergo axial loading [14].
Differences in the morphology of the vertebral bodies in human and guinea pig
is known that bones adapt to the action of mechanical forces by changing their morphology [15], so the size of the thoracic vertebrae in humans increases from the first to the last [16] and this trend continues distally so that the last lumbar vertebra has the greatest length, height and width [17]. On the other hand, guinea pig’s lumbar vertebrae have an irregular trapezoid geometry and the body lengths of L4 and L5 are the largest [18]. The diameter of the spinal canal is not uniform along its entire length and in humans its width increases from the first to the last lumbar vertebra [19]. Thus, in humans, there is a correlation between the diameter of the spinal canal and the place of greatest load on the spine, which can be considered a remarkable protective mechanism that aims to prevent pressure on the spinal cord that could occur when a higher mechanical force is applied. On the other hand, in guinea pigs, the greatest depth of the spinal canal was measured at the level of L4 [18].
Conclusion
Although some authors indicate that the spinal column of quadrupedal organisms (merino sheep) suffers a strong axial load [13], the results of morphometric studies of the spinal column of guinea pigs do not agree with the previous statement [18]. Namely, in guinea pigs L4 behaves atypically in relation to the other lumbar vertebrae and indicate that this level of the spinal column could be the highest load in these animals.
References
- Silvia FO, Alcantara D, Carvalho RC, Favaron PO, Santos AC, et al. (2016) Development of the central nervous system in guinea pig (Cavia porcellus, Rodentia, Caviidae). Pesq Vet Bras 36(8): 753-760.
- Mazensky D, Danko J, Petrovova E, Supuka P, Supukova A (2015) Anatomical study of the arterial blood supply to the thoracolumbar spinal cord in guinea pig. Ant Sci Int 90(4): 203-208.
- McDougall JJ, Andruski B, Schuelert N, Hallgrimsson B, Matyas JR (2009) Unravelling the relationship between age, nociception and joint destruction in naturally occurring osteoarthritis of Dunkin Hartley guinea pigs. Pain 141(3): 222-232.
- Guerner JN, Erulkar JS, Patel TC, Panjab MM (2000) Biomechanical evaluation of the New Zeland white rabbit lumbar spine: a physiologic characteristic. Eur Spine J 9(3): 250-255.
- Schulz KS, Waldron DR, Grant JW, Smith G, Shires PK (1996) Biomechanics of the thoracolumbar vertebral column of dogs during lateral bending. Am J Vet Res 57(8): 1228-1232.
- Wilke HJ, Geppert J, Kienle A (2011) Biomechanical in vitro evaluation of the complete porcine spine in comparison with data of the human spine. Eur Spine J 20(11): 1859-1868.
- Mageed M, Berner D, Jülke H, Hohaus C, Brehm W, et al. (2013) Is sheep lumbar spine a suitable alternative model for human spinal research? Morphometrical comparison study. ILAR J 29(4): 183-189.
- Riley LH, Eck JC, Yoshida H, Koh YD, You JW, et al. (2004) A Biomechanical comparison of calf versus cadaver lumbar spine models. Spine Ј 29(11): 217-220.
- Buttermann GR, Beaubien BP, Saeger LC (2009) Mature runt cow lumbar intradiscal pressures and motion segment biomechanics. Spine J 9(2): 105-114.
- Narita Y, Kuratani S (2005) Evolution of the vertebral formulae in mammals: A perspective on developmental constraints. J Exp Zool Part B 304(2): 91-106.
- Brocal J, Decker S, José LR, Guevar J, Ortega M, et al. (2018) Evaluation of radiography as a screening method for detection and characterisation of congenital vertebral malformations in dogs. Vet Rec 182(20): 573.
- Proks P, Johansen TM, Nývltová I, Komenda D, Cernochová H, et al. (2021) Vertebral formulae and congenital vertebral anomalies in guinea pigs: A Retrospective Radiographic Study. Animals 11(3): 589.
- Reitmaier S, Schmidt H, Ihler R, Kocak T, Graf N, et al. (2013) Preliminary investigations on intradiscal pressures during daily activities: an in vivo study using the merino sheep. PLoS ONE 8(7): e69610.
- Smit TH (2002) The use of a quadruped as an in vivo model for the study of the spine-biomechanical considerations. Eur Spine J 11(2): 137-144.
- Yavropoulou MP, Yovos JG (2016) The molecular basis of bone mechanotransduction. JMNI 16(3) 221-236.
- Goh S, Tan C, Price RI, Edmondston SJ, Song S, et al. (2000) Influence of age and gender on thoracic vertebral body shape and disc degeneration: An MR investigation of 169 cases. J Anat 197: 647-657.
- Tan SH, Teo EC, Chua HC (2004) Quantitative three-dimensional anatomy of cervical, thoracic and lumbar vertebrae of Chinese Singaporeans. Eur Spine J 13(2): 137-146.
- Mitrović MJ, Kitanović S, Tatalović N, Todorović A, Lazarević MM (2023) Radiological investigation of guinea pig (Cavia porcellus) lumbar vertebrae morphology- a biomechanical aspect. Acta veterinaria-Beograd 73(1): 55-70.
- Griffith JF, Huang J, Law SW, Xiao F, Leung JCS, et al. (2016) Population reference for development lumbar spinal canal size. Quant Imag Med Surg 6(6): 671-679.
© 2023 Marko Jumake Mitrović. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






