- Submissions

Full Text
Techniques in Neurosurgery & Neurology
A Pilot Study of Survival in Glioblastomas: Proliferative Index Ki-67, Risk Factors and Implications
Ricardo Augusto Delfino1*, Fernando Jacobsen de Barros1, Rafael Mourae Sucupira1, Marcelo Gomes de Almeida1, Gil Patrus Mundim Pena2, Isabel Cristina Gomes3, Augusto Afonso Guerra4, Brian Godman5,6,7,8 and Alessandra Maciel Almeida9
1Department of Neurosurgery, Madre Teresa Hospital, Brazil
2Department of Neuropathology, Madre Teresa Hospital, Brazil
3Post Graduation Program in Health Sciences, Faculty of Medical Sciences of Minas Gerais (FCM-MG), Brazil
4Post Graduation Program in Medicines and Pharmaceutical Assistance, Federal University of Minas Gerais (UFMG), Brazil
5Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, UK
6Division of Public Health Pharmacy and Management, Sefako Makgatho Health Sciences University, South Africa
7Department of Laboratory Medicine, Karolinska Institute, Karolinska University, Sweden
8School of Pharmaceutical Sciences, University Sains Malaysia, Penang, Malaysia
9Post-Graduation Program in Health Sciences, Faculty of Medical Sciences of Minas Gerais (FCM-MG), Federal University of Minas Gerais (UFMG), Brazil
*Corresponding author:Ricardo Augusto Delfino, Department of Neurosurgery, Madre Teresa Hospital, Belo Horizonte, Minas Gerais, Brazil; Post-Graduation Program in Health Sciences- Faculty of Medical Sciences of Minas Gerais (FCMMG)
Submission: May 17, 2023;Published: May 31, 2023

ISSN 2637-7748
Volume5 Issue3
Abstract
Glioblastoma is the most frequent and aggressive primary malignant brain tumor in adults. It is considered a fatal disease, and the average survival time is approximately 12 to 18 months.
Objectives: Evaluate risk factors and the survival time in patients diagnosed with glioblastoma.
Materials and methods: A retrospective study was performed at a neurosurgery reference hospital in
Belo Horizonte, MG, Brazil observing patients who underwent glioblastoma surgery. From the electronic
medical records were collected the sociodemographic data, Karnofsky Performance Status (KPS), tumor
location and volume. Surgical specimens were evaluated according to protein expression using the Ki-67
index and the date of death was evaluated in the Mortality Information System.
Results: The sample consisted of 25 patients, most were male (52%) and 40% were older than 65 years;
76% of patients had a KPS index>80. The tumor location was predominantly frontal and temporal. Longer
mean survival observed among males, patients <65 years, Ki-67 index up to 20%, KPS > 80 and frontal
tumor location. A reduction in the risk of dying was observed in patients with a KPS > 80. Patients who
survived less than 12 months had a higher median Ki-67 index.
Conclusion: No statistically significant differences were found in the survival curves comparing a Ki-67
index up to 20 and Ki-67 index > 20 or the stratified Ki-67 index, however, higher risk of dying in older
patients with a Ki-67 index > 20.
Keywords: Glioblastoma; Ki-67; Immunohistochemistry; Survival
Introduction
Glioblastoma (GB), also called high-grade glioma or grade IV glioma, is the most common and aggressive primary malignant brain tumor of the Central Nervous System (CNS) and is more frequent in patients older than 65 years [1-7]. Glioblastoma currently accounts for 12-15% of all intracranial tumors and represents more than 50% of all glial tumors [1,3-5,8]. Glioblastomas /grade IV gliomas represents 60-70% of all gliomas diagnosed, with an incidence rate of 3.2 to 5.3 per 100.000 people [8,9]. The highest incidence rates are found in European countries. In Brazil, in 2015, there were 4,718 deaths from GB, and 4,315 deaths in women [10]. In general, these tumors are often difficult to cure surgically and may also have some resistance to radiation and chemotherapy [11,12].
Despite the recommended multimodal treatment, including surgery, radiotherapy, and chemotherapy the average survival time varies between 12 and 18 months after the initial diagnosis [2,5,13,14]. Although survival rates have increased since 2005, before the landmark trial of radiotherapy plus concomitant and adjuvant temozolamide, they still remain significantly limited, with reported 2-year survival of 20% and only 3% at 5 years [5]. Many factors may influence the survival of these patients. These include tumor location, age, extent of surgical resection, Karnofsky Performance Scale (KPS) score, methylation state of O-6- methylguanine-DNA methyltransferase, progression free survival, potentially neutrophil-to-lymphocyte ratios and IDH mutation status [7,13,15,16]. Furthermore, certain genetic abnormalities and the probability of the presence of other tumor markers may be correlated with greater aggressiveness [17].
Due to the heterogeneity of the nature of GB and as an attempt to individualize treatment strategies, several tumor markers have been used and have been shown to be directly related to the prognosis of these patients [11].
The Ki-67 protein is a cellular marker associated with ribosomal RNA transcription (rRNA) and therefore cell proliferation. It can be detected during all active phases of the cell cycle (G1, S, G2 and M phase) and is not detected in quiescent cells (G0) or in the initial stage of G1 phase [18]. It has been used to assess the proliferative activity of several tumors, including those of the CNS [19-21] and it has been suggested as a tool to estimate the growth fraction of any human cell population [22]. Ki-67 antigen positivity may reflect the ability of the cell to continue proliferating after tumor resection, and as mentioned, has been considered a reliable indicator for the activity of tumor cell proliferation and it is associated with histological grade in gliomas [23]. Moreover, Ki-67 antigen positivity has been shown to be correlated with patient survival, in addition to being a tool for estimating tumor class malignancy and patient prognosis [24,25]. The objective of this study was to evaluate the factors associated with survival in patients diagnosed with GB who underwent surgery and standardized treatment and potentially verify the association between the Ki-67 index and survival.
Material and Methods
This pilot study involved a retrospective cohort of patients diagnosed with grade IV gliomas (glioblastomas) who underwent surgery at a neurosurgery reference hospital in Belo Horizonte, Minas Gerais, Brazil, between January 2008 and December 2014. The selection of the period is related to survival follow-up that could extrapolate up to 5 years (December 2019). Patients were selected with a diagnosis of grade IV gliomas confirmed by histological analysis who underwent surgery involving total or subtotal resection (greater than 90% of excision of the total tumor mass) with the same combined radiotherapy and standard oral chemotherapy (temozolomide), a standardized protocol of the European Association for Neuro-Oncology [26]. The dosage of fractional RT used was 60Gy in fractions of 1.8-2.0Gy given on weekdays for six weeks (about 30 sessions) with concomitant oral Temozolomide (TMZ) capsules, administered at 75mg/m2 per day during this period, followed by 12 months of adjuvant TMZ administered on days one to five of each 28-day cycle [26].
Patients who were only subjected to tumor biopsy or partial tumor resection (< 90% of the initial tumor volume) and those with tumor recurrence, tumoral progression, prior neurosurgical treatment and with alternative oncological treatment were excluded in order to have a more homogeneous patient population.
Electronic data were collected from the medical records including sociodemographic data (age, gender, mother’s name, municipality and state of residence), date of surgery (date of diagnosis), degree of tumor resection (total, subtotal, partial, or biopsy) and evaluation of the degree of functional impairment. KPS) is routinely measured in this hospital in oncology patients and was used to assess the degree of functional impairment in the final cohort of patients. KPS is related to independence regarding self-care, the ability to perform daily activities and active work, and the need for assistance, and ranges from 0 to 100. Values between 80 and 100 indicate that patients are functionally normal, able to perform normal activities and work and do not require assistance, while values between 50 and 70 indicate that patients are incapable of work but are able to live at home and perform their daily activities with varying amounts of need for assistance [27]. The study had no need for informed consent, as it was carried out by retrospective analysis of electronic files, preserving patients’ confidentiality
The location of the tumor in the brain and its laterality were determined by imaging (magnetic resonance) examinations that were routinely performed preoperatively, and these data were obtained from the electronic records of the radiology department of the hospital. The tumor mass volume was calculated using the ABC/2 formula proposed to calculate the volume of intracerebral hemorrhage. The technique is derived from an approximation according to the formula for ellipsoids in which A is the largest hemorrhage diameter, B is 90° from the diameter of A and C is the approximate number of Computed Tomography (CT) sections with hemorrhage multiplied by the thickness of the cuts, which is a standard measure in neurosurgical practice [28]. The formula was used to estimate tumor volume in an analogous manner considering the anteroposterior, medial-lateral and cranial-caudal intervals and magnetic resonance imaging was performed routinely in the pre and postoperative period in order to estimate the degree of tumor resection.
The immunohistochemical evaluation of the surgical specimens was performed at the reference hospital’s pathological anatomy service, and only conclusive analyses were considered, according to the quality of analysis and sample representativeness. Surgical specimens were evaluated according to protein expression using Ki-67 index. Evaluation of the Ki-67 protein was performed with the immunohistochemical technique of streptavidin-biotin-peroxidase, followed by the quantitative analysis of positive elements by manual counting, as a standard measurement [29]. Evaluation of other molecular markers, such as IDH1 or MGMT promotor methylation status test were not included in the study because there was no way to standardize the entire sample for analysis. These tests were only undertaken in specific cases due to the difficulty in covering treatment costs by health insurers.
Death dates were obtained from the Mortality Information System (Sistema de Informação de Mortalidade - SIM) based on the variables (name, age, gender, mother’s name, municipality and state of residence) [30]. Survival time was determined by the time that had elapsed between the date of surgery and the date of death, or last follow-up date in case of survival at the end of the study period. Patient’s ages were recorded as younger patients with higher performance scores do appear to have longer survival rates [31], with age significantly associated with survival after diagnosis for all gliomas , but the effect is more pronounced in GBM. Patients were classified according to the following demographic and clinical characteristics: gender, age (< 65 years, 65 years or more), residence (in the capital or outside the state capital), Ki-67 index (up to 20% or > 20%), localization in the brain (frontal, occipital, parietal, or temporal), tumor volume (in cm3), KPS status (up to 80 or > 80), and percentage mortality in line with previous studies.
Qualitative variables are expressed as absolute and relative frequencies. Quantitative variables, after being subjected to Shapiro-Wilk’s normality test, are expressed as the mean ± Standard Deviation (SD), in the case of normality, and the median ± interquartile range otherwise. Comparison of quantitative variables between two groups was performed using the Wilcoxon Mann-Whitney test for independent samples. The survival time until death was evaluated with Kaplan-Meier curves using the logrank test for group comparisons. A multivariate Cox model was constructed, and the results are presented as the Hazard Ratio (HR) and respective confidence interval. The quality of fit was assessed using the Wald test and the risk proportionality assumption using Schoenfeld residuals. The analyses were performed in the free program R version 3.5.1 and were considered significant at p < 0.05.
Result
In the study period, a total of 118 patients with grade IV gliomas / GB, confirmed by histological analysis, underwent surgical treatment at the hospital and underwent the same combined radiotherapy and standard oral chemotherapy. From these, were performed a total of 81 partial resections/biopsies (first group) and 37 total or subtotal resections were performed (second group). As mentioned, we initially selected for the analysis only 37 patients who underwent total or subtotal resection (at least 90% resection of the initial tumor volume), from the second group; of these 37 patients, 10 patients were subsequently excluded who had undergone previous neurosurgery and 2 were excluded due to tumor recurrence. This left finally 25 patients for the study who had surgical conditions of wide excision.
The final sample of 25 patients consisted of 13 male patients (52%) and 12 female patients (48%), with 40% 65 years of age or older (mean age: 60.8±13.6 years). The average survival time was 9 months. The majority of the individuals resided in Belo Horizonte/ MG (64%). The median tumor volume was 24.2 ± 28.5. The tumor location was predominantly frontal and temporal (32% for both), followed by parietal (20%). The majority of patients had a KPS status > 80 (76%). Eighty-eight percent of patients died (Table 1). Regarding the evaluation of the protein expression in the surgical specimens, 68% had a Ki-67 index > 20% (median 26.4±20).
Table 1:Demographic and clinical characteristics of the sample.
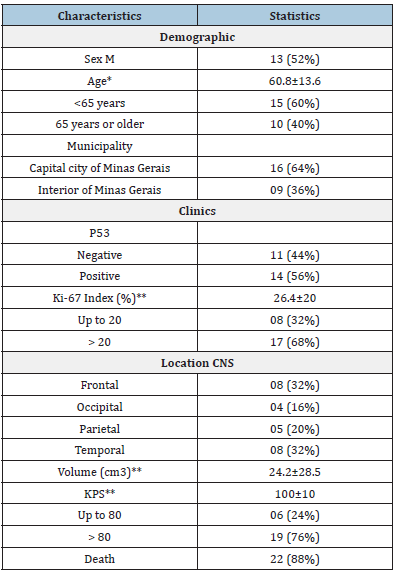
*Data presented as mean±SD; **Data presented as median±DI; CNS: Central Nervous System, KPS: Karnofsky Performance Scale.
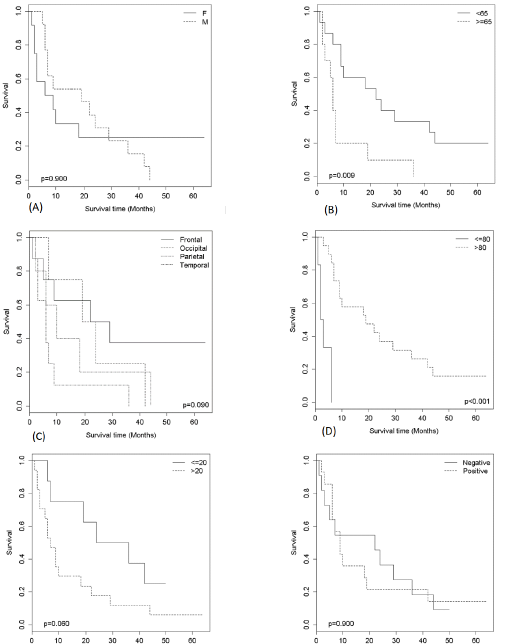
Considering death as an event of interest, the estimate of survival was stratified by gender (a), age (b), tumor location (c), KPS status (d) and Ki-67 index (e). The estimate was obtained using the Kaplan-Meier estimator. Patients aged < 65 years with a KPS > 80 had significantly prolonged survival (p = 0.009 and p = 0.001, respectively, log-rank test). Regarding the Ki-67 index, better survival was observed with a Ki-67 index up to 20%; however, the difference was not statistically significant (p = 0.06) (Figure 1). When the Ki-67 index was stratified (up to 20, 20-25, 26-30 and >30), no statistically significant differences were observed with respect to survival (p = 0.300).
Figure 1:Kaplan-Meier curves according to (a), sex, (b)age, (c) location, (d) Karnofsky, (e) Ki-67 and (f) p53. The
p-values refer to the Log-Rank test.
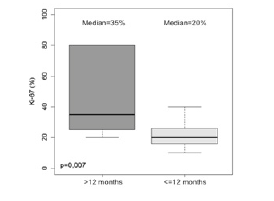
The median survival time obtained by the Kaplan-Meier estimator showed higher averages for males (19 months), patients < 65 years (22 months), those with a Ki-67 index up to 20% (30 months), those with a KPS > 80 (16 months) and those with tumors in a frontal location (25.5 months) followed by those with tumors in an occipital location (21.5 months) (Table 2). The results of the multiple analysis using the Cox model showed that in relation to the age variable, patients 65 years of age or older had a risk of dying (HR) that was 10.395 times higher than that of those aged less than 65 years (p < 0.001). For the Ki-67 index, the risk of dying (HR) was 8.346 times higher in patients with values greater than 20 than in those with values up to 20 (p < 0.001). For the KPS status, patients with a KPS status > 80 had a reduced risk of dying (HR) of 0.041 compared with those with a KPS status up to 80; thus, KPS status was considered a protective factor (p = 0.002) (Table 3). Wald test (p<0.001), R2 = 0.718. There was no breach of the proportional risks assumption, for the evaluation of schoenfeld residuals. KPS: karnofsky Performance Scale. Figure 2 compares the median survival of patients with a Ki-67 index (%) in patients who survived less than 12 months with those who survived more than 12 months. Patients who survived less than 12 months had a higher median Ki-67 index than those who survived more than 12 months (p = 0.007).
Table 2:Median survival time obtained by the Kaplan- Meier estimator according to characteristics.
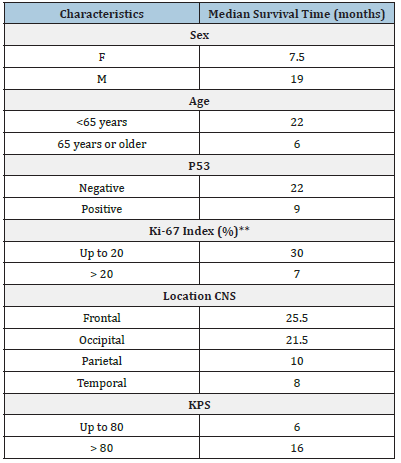
Table 3:Cox model for the survival time until death.
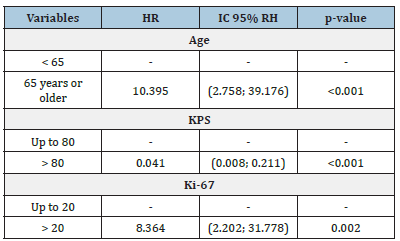
Figure 2:Boxplot values of Ki-67 (%) according to
survival time. The p-value refers to the Wilcoxon Mann-
Whitney test for independent samples.
Discussion
The multivariate analysis model showed a higher risk of dying in older patients with a Ki-67 index > 20. This effect of age on survival in patients with GB has been seen in other studies [2- 4,8]. Overall, younger patients with a good initial KPS assessment had longer survival times in our study, similar to other studies [32-34]. Patient age and KPS score are now widely recognized as prognostic factors, and younger patients with higher performance scores have longer survival rates [7,31]. Age is also significantly associated with survival after diagnosis for all gliomas, but the effect is more pronounced in GB [31]. Pan et al. [34] in a cohort of 14,675 adult patients with GB also found better survival times in younger patients. Similarly, Lacroix et al. [32] when evaluating 416 patients with GB observed greater survival in younger patients with a preoperative KPS score > 80, with Abdullah et al. [33] also showing improved survival in patients with higher KPS scores.
The present study investigated the association of the Ki-67 index with the survival of patients with grade IV gliomas, also called glioblastoma. Considering 20% as the cutoff point for the Ki-67 index, in this study, the difference in the survival curves was not statistically significant; however, patients who survived less than 12 months had a higher median Ki-67 index, and the multivariate analysis model showed a higher risk of dying in older patients with a Ki-67 index > 20. Miconi et al. [35] showed that positive tumor stem cells have been associated with an increased Ki-67 index, directly related to worse survival. However, there seems to be conflicting evidence about the association between the Ki-67 index and overall survival in GB [36], There are continuing debates in the literature regarding its use as a prognostic factor in patients with gliomas. The mechanism underlying the effect of Ki- 67 index protein expression on tumor progression and prognosis remains uncertain [22]. Low or high levels of Ki-67 expression are directly associated with grade II-III or IV gliomas; consequently, Ki- 67 expression might be considered a prognostic factor in patients with grade II-III gliomas [37], but its use as a prognostic indicator for patients with glioblastoma remains unclear [38]. Nevertheless, several studies have shown that the evaluation of markers of cell proliferation, such as Ki-67 index, are considered useful to predict survival [25,36,39].
A study conducted in China evaluated the prognostic value of Ki-67 index expression in 156 Chinese patients with GB and found that Ki-67 index was an independent prognostic indicator. Survival analysis of Ki-67 index overexpression revealed an association with worse clinical outcome (progression-free survival (p = 0.010) and overall survival (p = 0.007) [40]. Other studies have also concluded that the greater Ki-67 index, the greater the survival, including a study conducted in Vienna in 114 patients with GB between 1995 and 1999, which considered cutoff points of the Ki-67 index ≤27% or > 27 [41]. A study conducted in Sidney from 2002 to 2012 involving 71 patients with GB also showed that there was a statistically significant correlation between the Ki-67 index and overall survival. In patients with a Ki-67 index > 22%, 5-year survival was approximately 30% compared with 5% in patients with a Ki-67 index ≤22% [36].
Stopschinski et al. [11] found that the co expression and proportion of CD133-positive cells and Ki-67 index in vivo were correlated with a worse prognosis and observed a consistent correlation between CD133/Ki-67 index co expression and the generation of tumor stem cells with an important impact on survival. Thotakura et al. [25] described the association of a higher Ki-67 index in primary astrocytomas according to the progression of the degree of classification between grade I (mean = 3.36±4.59) up to grade IV (mean = 38.7±7.19) [25]. Cell wall alterations were also observed on an electron microscope analysis of GB tumor cells using the Ki-67 index, and it is considered an important prognostic marker in human astrocytomas. Sarkisian et al. [39] observed the presence of primary cilia, organelles that can facilitate tumor proliferation, migration, and survival of these cancer cells, suggesting that the Ki-67 index may be related to both the presence of replicating tumor stem cells and cell wall changes.
On the other hand, a study that evaluated 75 patients with GB in Russia did not reveal a statistically significant association of the Ki-67 index, classified as high or low expression, with the prognosis of the disease. Even for Ki-67 index, they verified low prognostic value of this molecular marker in patients with high-grade GB, or glioblastoma grade IV [38]. A meta-analysis of 51 studies involving a total of 4,307 patients sought to clarify the impact of the Ki-67 index on the survival of patients with any grade glioma and evaluated the clinical characteristics, overall and progression-free survival, and Ki-67 expression at different times. The results showed that the overexpression of Ki-67 index was able to predict worse overall and progression-free survival in patients with gliomas (p = 0.000) regardless of the region, type of pathology, and statistical method. However, only 23 studies included patients with GB, and of these, ten studies included patients with GB but there was considerable cutoff variation for Ki-67 index (1.5% - 35%). Seven of these studies demonstrated a statistically significant difference in the Ki-67 index with progression-free survival, and no study showed a difference in overall survival [22].
The limitation of this study was the reason to standardize the samples considering only patients who underwent total or subtotal resection (more than 90% of the excision) As a result, a large number of patients treated at the hospital in the same study follow-up period were excluded because they were only biopsied or partially treated, or because they were recurrent tumors. Another limitation was the lack of ability to analyze other molecular markers including IDH1 or the MGMT promotor methylation status. Despite these limitations, we believe our findings are robust adding to the literature in this area to guide future management strategies. Recent advances in glioblastoma research have showed relationship with the silent information regulator proteins (Sirtuins, or SIRTs) and initiation as well as in the progression of GB, signalizing emerging treatment targets and maybe will provide novel prospective studies in this field [42].
Conclusion
The present study investigated the association of the Ki-67 index with the survival of patients with GB. Considering death as an event of interest, the estimate of survival was stratified by gender, age, tumor location, KPS status and Ki-67 index. The median survival time showed higher averages for males, patients < 65 years, Ki-67 index up to 20%, KPS > 80 and with tumors in a frontal location. The results of this study showed better survival for male patients aged < 65 years with a KPS score > 80. However, no statistically significant difference was found in the survival curves comparing a Ki-67 index of up to 20 and a Ki-67 index > 20 and a stratified Ki-67 index (up to 20, 20-25, 26-30, and > 30), although a higher risk of dying was found in older patients with a Ki-67 index > 20. Patients who survived more than 12 months had a lower median Ki-67 index. Overall, these findings contribute to strengthening the evidence for the use of the Ki-67 index as a prognostic tumor marker, which is an affordable and low-cost method. However, more studies are needed to define an ideal cutoff value for the Ki-67 index in patients with GB. The progressive improvement of access to other molecular markers will also aid better prognostic assessment of the disease in the future to further improve patient management.
Ethical Approval
The study was approved by the Research Ethics Committee of the Lucas Machado Educational Foundation (Fundação Educacional Lucas Machado - FELUMA) with CAEE no. and opinion number CAAE 81071517.4.0000.5134 of February 20, 2018.
References
- Sarkar A, Chiocca EA (2012) Glioblastoma and malignant astrocytoma. In: Brain Tumors.
- Ohgaki H, Kleihues P (2005) Epidemiology and etiology of gliomas. Acta Neuropathol 109(1): 93-108.
- Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee SU (2017) Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pacific J Cancer Prev 18(1): 3-9.
- Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, et al. (2014) The epidemiology of glioma in adults: A state of the science review. Neuro Oncol 16(7): 896-913.
- Poon MC, Sudlow CM, Figueroa JD, Brennan PM (2020) Longer-term (≥2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: a systematic review and meta-analysis. Sci Rep 10(1): 1-10.
- Taslimi S, Ye V, Wen P, Zadeh G (2021) Lessons learned from contemporary glioblastoma randomized clinical trials through systematic review and network meta-analysis: part 2 recurrent glioblastoma. Neuro-Oncology Adv 3(1): 1-10.
- Lei Y, Li Y, Hu Q, Wang J, Sui AX (2019) Prognostic impact of neutrophil-to-lymphocyte ratio in gliomas: A systematic review and meta-analysis. World J Surg Oncol 17(1): 1-8.
- Tamimi AF, Juweid M (2017) Epidemiology and outcome of glioblastoma, pp. 143-153.
- Stewart B, Wild C (2014) World Cancer Report 2014. Int Agency Res Cancer.
- José Alencar Gomes da Silva (2016) National Cancer Institute. INCA-National Cancer Institute-Estimate 201. Ministry of Health National Cancer Institute José Alencar Gomes da Silva, p. 124.
- Stopschinski BE, Beier CP, Beier D (2013) Glioblastoma cancer stem cells-from concept to clinical application. Cancer Lett 338(1): 32-40.
- Bischof J, Westhoff M, Wagner JE, Halatsch M, Trentmann S, et al. (2017) Cancer stem cells: The potential role of autophagy, proteolysis, and cathepsins in glioblastoma stem cells. Tumor Biol 39(3): 1010428317692227.
- Tykocki T, Eltayeb M (2018) Ten-year survival in glioblastoma. A systematic review. J Clin Neurosci 54: 7-13.
- Hillembrand LM, Wijesekera O, Meade PS, Mampre D, Jackson C, et al. (2020) Trends in glioblastoma: Outcomes over time and type of intervention: A systematic evidence based analysis. J Neurooncol 147(2): 297-307.
- Theeler BJ, Gilbert MR (2015) Advances in the treatment of newly diagnosed glioblastoma. BMC Med 13(1): 1-11.
- Lu VM, O’Connor KP, Shah AH, Eichberg DG, Luther EM, et al. (2020) The prognostic significance of CDKN2A homozygous deletion in IDH-mutant lower-grade glioma and glioblastoma: A systematic review of contemporary literature. J Neurooncol 148(2): 221-229.
- Paldor I, Pearce FC, Drummond KJ, Kaye AH (2016) Frontal glioblastoma multiforme may be biologically distinct from non-frontal and multilobar tumors. J Clin Neurosci 34: 128-132.
- Fakhrjou A, Tabrizi AD, Ghojazadeh M, Ghorashi S, Velayati A, et al. (2013) Diagnostic value of protein Ki67 (MIB-1) in atypical pap smears of postmenopausal women. Asian Pac J Cancer Prev 14(8): 4815-4818.
- Szopa W, Burley TA, Marek GK, Kaspera W (2017) Diagnostic and therapeutic biomarkers in glioblastoma: Current status and future perspectives. Biomed Res Int 2017: 8013575.
- Preusser M, Heinzl H, Gelpi E, Höftberger R, Fischer I, et al. Ki67 index in intracranial ependymoma: A promising histopathological candidate biomarker. Histopathology 53(1): 39-47.
- Stoyanov GS, Dzhenkov DL, Kitanova M, Donev IS, Ghenev P (2017) Correlation between Ki-67 index, world health organization grade and patient survival in glial tumors with astrocytic differentiation. Cureus 9(6): e1396.
- Chen WJ, He DS, Tang RX, Ren FH, Chen G (2015) Ki-67 is a valuable prognostic factor in gliomas: Evidence from a systematic review and meta-analysis. Asian Pac J Cancer Prev 16(2): 411-420.
- Hu X, Miao W, Zou Y, Zhang W, Zhang Y, et al. (2013) Expression of p53, epidermal growth factor receptor, Ki-67 and O6-methylguanine-DNA methyltransferase in human gliomas. Oncol Lett 6(1): 130-134.
- Yábar A, Meléndez R, Muñoz S, Deneo H, Freire J, et al. (2017) Effect of Ki-67 assessment in the distribution of breast cancer subtypes: Evaluation in a cohort of Latin American patients. Mol Clin Oncol 6(4): 503-509.
- Thotakura M, Tirumalasetti N, Krishna R (2014) Role of Ki-67 labeling index as an adjunct to the histopathological diagnosis and grading of astrocytomas. J Cancer Res Ther 10(3): 641-645.
- Weller M, Bent M, Tonn JC, Stupp R, Preusser M, et al. (2017) European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol 18(6): e315-e329.
- Schag CC, Heinrich RL, Ganz PA (1984) Karnofsky performance status revisited: Reliability, validity, and guidelines. J Clin Oncol 2(3): 187-193.
- Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, et al. (1996) The ABCs of measuring intracerebral hemorrhage volumes. Stroke 27(8): 1304-1305.
- Labit CB, Chinot O, Ochi C, Gambarelli D, Dufour H, et al. (1998) Prognostic significance of Ki67, p53 and epidermal growth factor receptor immunostaining in human glioblastomas. Neuropathol Appl Neurobiol 24(5): 381-388.
- Brasil MD, Saúde FN (2001) Mortality Information System Procedure Manual. Man System Procedures Mortality Information.
- Lacroix M, Said DA, Fourney DR, Gokaslan ZL, Shi W, et al. (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg 95(2): 190-198.
- Abdullah KG, Ramayya A, Thawani JP, Macyszyn L, Lage MM, et al. (2015) Factors associated with increased survival after surgical resection of glioblastoma in octogenarians. PLoS One 10(5): e0127202.
- Pan IW, Ferguson SD, Lam S (2015) Patient and treatment factors associated with survival among adult glioblastoma patients: A USA population-based study from 2000-2010. J Clin Neurosci 22(10): 1575-1581.
- Miconi G, Palumbo P, Dehcordi SR, Torre CL, Lombardi F, et al. (2015) Immunophenotypic characterization of human glioblastoma stem cells: Correlation with clinical outcome. J Cell Biochem 116(5): 864-876.
- Wong E, Nahar N, Hau E, Varikatt W, Gebski V, et al. (2018) Cut-point for Ki-67 proliferation index as a prognostic marker for glioblastoma. Asia Pac J Clin Oncol 15(1): 5-9.
- Yuan Y, Xiang W, Yanhui L, Ruofei L, Shuang L, et al. (2013) Ki-67 overexpression in WHO grade II gliomas is associated with poor postoperative seizure control. Seizure 22(10): 877-881.
- Tsidulko AY, Kazanskaya GM, Kostromskaya DV, Aidagulova SV, Kiselev RS, et al. (2017) Prognostic relevance of NG2/CSPG4, CD44 and Ki-67 in patients with glioblastoma. Tumor Biol 39(9): 1010428317724282.
- Sarkisian MR, Siebzehnrubl D, Minh LH, Deleyrolle L, Silver DJ, et al. (2014) Detection of primary cilia in human glioblastoma. J Neurooncol. 117(1): 15-24.
- Jin Q, Zhang W, Qiu XG, Yan W, You G, et al. (2011) Gene expression profiling reveals Ki-67 associated proliferation signature in human glioblastoma. Chin Med J (Engl) 124(17):2584-2588.
- Bredel M, Piribauer M, Marosi C, Birner P, Gatterbauer B, et al. (2002) High expression of DNA topoisomerase IIalpha and Ki-67 antigen is associated with prolonged survival in glioblastoma patients. Eur J Cancer 38(10): 1343-1347.
- Kunadis E, Piperi C (2022) Exploring the multi-faceted role of sirtuins in glioblastoma pathogenesis and targeting options. Int J Mol Sci 23(21): 12889.
- Martins IJ (2016) Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Advances in Aging Research 5(1): 9-26.
© 2023 Ricardo Augusto Delfino. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






