- Submissions

Full Text
Techniques in Neurosurgery & Neurology
Vici Syndrome: Neuroimaging and Clinical Findings
Yannic A Gretener, Thierry AGM Huisman, Nilesh K Desai and Stephen F Kralik*
Division of Neuroradiology, Department of Radiology, Texas Children’s Hospital and Baylor College of Medicine, USA
*Corresponding author:Stephen F Kralik, Edward B Singleton Department of Radiology, Texas Children’s Hospital and Baylor College of Medicine, 6701 Fannin Street, Suite 470, Houston, TX 77030, USA
Submission: February 23, 2023;Published: March 09, 2023

ISSN 2637-7748
Volume5 Issue3
Abstract
Vici syndrome is a rare autosomal recessive multisystemic disorder secondary to a mutation in the EPG5 gene. The syndrome is characterized by brain malformations, the most common being corpus callosum agenesis, followed by pontine hypoplasia, and white matter hypomyelination. Clinical findings may include cataract, developmental delay, seizures, feeding disorders, cardiomegaly, and recurrent infections due to immunodeficiency. This report familiarizes the radiologist with clinical and imaging findings in Vici syndrome.
Introduction
Vici syndrome (OMIM 242840) is an extremely rare autosomal recessive disease with less than 80 confirmed cases published to date. Vici is caused by autosomal recessive mutations of the EPG5 gene on chromosome 18q. The protein encoded by the EPG5 gene is essential for systemic autophagy processes. Mutations of the EPG5 gene result in the production of deficient EPG5 proteins with a subsequent impaired autophagy and immune response to infectious antigens [1]. It is usually diagnosed in the first two years of life. Median survival time is 24 months [2]. In Vici syndrome, multiple systems can be affected. Involvement of the central nervous system include corpus callosum malformations, pontine hypoplasia, and white matter hypomyelination which are clinically linked to profound global neurocognitive and functional developmental delays. In addition, cataracts (the most frequent ocular abnormality), oculo-cutaneous hypopigmentation and cardiomyopathy may be seen. Less frequently, seizures, hearing loss, maxillofacial clefts and impaired function of the thyroid gland, liver and kidneys as well as postnatal growth retardation are noted. Affected children may acquire a social smile, some degree of head control, and the ability to roll over, however there have been no reports of children sitting independently [3]. The syndrome is named after Dr. Carlo Vici who described the constellation of findings in two siblings in 1988 [4]. The goal of this manuscript is to report the clinical and neuroimaging findings in a 17-month-old child diagnosed with Vici-syndrome. The findings will be discussed in the context of the available literature.
Case Description
We report on a one-year-old Indian boy born in the United States. The patient’s paternal and maternal great grandfathers are brothers; his parents are second degree cousins. The child was born premature at 34-week gestational age by spontaneous, vacuum assisted vaginal delivery. He weighed 1.9kg at delivery. At 11-days of age, he was transferred to a tertiary children’s hospital because of feeding difficulties, stridor and concern for recurrent aspiration. Physical examination revealed congested nasal cavities but a structurally normal airway. A fiberoptic endoscopic evaluation of swallowing test (FEES) identified minor post cricoid residue with reflux but no laryngomalacia or obvious aspiration. Due to continued feeding difficulties, MRI of the brain was performed to rule out central nervous system abnormalities. Complete agenesis of the corpus callosum, in combination with a hypoplastic pons and mild inferior cerebellar hypoplasia was noted (Figure 1). Despite detailed initial clinical and genetic work up (chromosomal microarray), no definite diagnosis was made during the NICU hospitalization. Symptomatic, supportive treatment included placement of a gastrostomy tube in the NICU. Next to the neuroimaging findings, bilateral cataract, hip dysplasia, developmental delay, oral aversion, and high risk of skin breakdown was noted. On follow up, laryngomalacia was diagnosed. At 6 months of age bilateral cataract surgery was performed. After the child was transferred to our tertiary children’s hospital the constellation of clinical and radiographic findings, including recurrent infections raised the possibility of Vici syndrome.
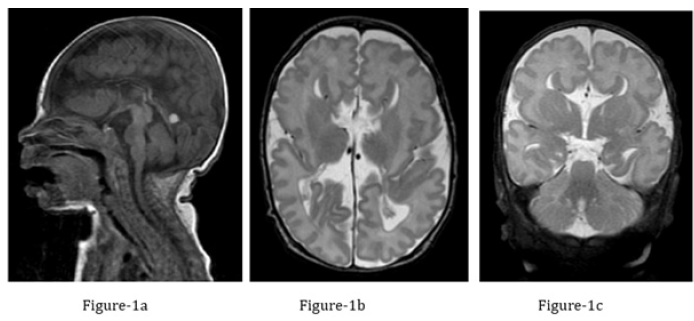
Figure 1:Brain MRI performed at one month of age.
a) Midline sagittal T1-weighted MR image of the brain: agenesis of the corpus callosum. The cerebellar vermis is well
formed, the pons is hypoplastic/flattened. An incidental residual, retracting extra-axial hematoma is noted superior
to the tentorium cerebelli.
b) Coronal T2-weighted MR image of the brain: Agenesis of the corpus callosum with classical “longhorn appearance”
of the lateral ventricles. Incomplete inversion of the cingulate gyri. T2-hypointense Probst bundles are noted along
the mesial contour of the lateral ventricles. The collateral sulci of the anteromedial temporal lobes demonstrate
dysgyria with craniocaudally deep sulci as well as malrotation of both hippocampi.
c) Axial T2-weighted MR image: Agenesis of the corpus callosum with mild volume reduction of the central white
matter. In addition mildly deformed posterior lateral ventricles as well as dysgyria of the Sylvian fissures which are
elongated in an AP direction.
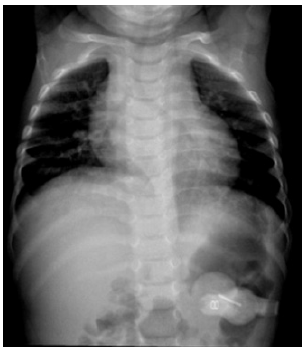
Seven out of the eight child’s presenting symptoms and imaging findings are indicators of the Vici syndrome [3]. Data from the subsequent comprehensive testing revealed a homozygous variant of uncertain significance (VUS) on the EPG5 gene associated with the Vici syndrome as well as a homozygous pathogenic variant in the Dymeclin (DYM) gene which encodes for the dymeclin protein. This EPG5 protein is believed to play a role in early brain development as well as Golgi-associated secretory pathways that are essential to endochondral bone formation. DYM mutations are also associated with two types of osteochondrodysplasic including the Dyggve- Melchior-Clausen dysplasia and the Smith-McCort dysplasia [5]. Based upon the clinical presentation, the imaging findings and the genetic work-up, the child was diagnosed with a combination of the Vici syndrome and the Dyggve-Melchior-Clausen syndrome, which explains the additional hip dysplasia. A follow up chest radiography at 1 year of life showed mild cardiomegaly, which is also a symptom of Vici syndrome (Figure 2). On follow up, the child had multiple visits at our hospital because of different diseases, including Covid-19 as well as several episodes of upper extremity stiffening and concerns of infantile spasms. Based upon the matching EEG studies he was diagnosed with tonic-clonic seizures.
Figure 2:Anterior-posterior radiography of the chest:
Mild cardiomegaly.
Discussion
Vici syndrome is a rare autosomal disease, first described in two siblings by Dr. Carlo Vici [4] Vici syndrome is caused by a mutation of the EPG5 gene, which hinders the process of autography impacting brain development and immuno-competency [2]. Multiple systems or organs may be affected (e.g., cataract) [3]. Most children die within 24 months of diagnosis [1].
In addition to genetic testing and clinical evaluation, neuroimaging is a helpful tool in diagnosing Vici syndrome. The most common imaging finding is agenesis of the corpus callosum. In each reported case, in which either an MRI or a transfontanellar ultrasound was performed, a corpus callosum agenesis could be identified. In 65% of reported cases a cardiomyopathy was documented, a chest radiography may be helpful to identify said cardiomyopathy [6]. Because corpus callosum agenesis is a rather non-specific imaging finding that may be seen in a vast array of brain malformations and syndromes familiarity with the Vici syndrome and its diverse clinical symptoms, it is essential for the (neuro-) radiologist to be familiar with this rare syndrome to narrow down the differential diagnosis. Recurrent infections should raise the suspicion for the final diagnosis. In conclusion, the combination of a corpus callosum agenesis, cardiomegaly, cataract, recurrent infections, developmental delay and feeding disorders secondary to a systemic immuno-deficiency should raise the suspicion of a Vicisyndrome. Genetic evaluation identifying an EPG5 gene mutation are confirmatory.
References
- Ehmke N, Nima P, Peter K, Mahmoud-R, Parviz K, et al. (2014) First description of a patient with vici syndrome due to a mutation affecting the penultimate exon of EPG5 and review of the literature. Am J Med Genet A 164(12): 3170-3175.
- Dafsari HS, Ebrahimi F, Saffari A (2022) EPG5-Related Disorder. Gene Reviews, University of Washington, Seattle, Washington, USA.
- Byrne, Susan, Carlo D, Mathias G, Heinz J (2016) Vici syndrome: A review. Orphanet Journal of Rare Diseases 11(1).
- Vici C, G Sabetta, M Gambarara, F Vigevano, E Bertini, et al. (1988) Agenesis of the corpus callosum, combined immunodeficiency, bilateral cataract, and hypopigmentation in two brothers. Am J Med Genet 29(1): 1-8.
- Denais C, Carolyn L, Laura S, Jacqueline H, Dimitra D et al. (2010) Dymeclin, the gene underlying dyggve-melchior-clausen syndrome, encodes a protein integral to extracellular matrix and Golgi organization and is associated with protein secretion pathways critical in bone development. Human Mutation 3(2): 231-239.
- Alzahrani A, Abdulrahman A, Rahaf W (2018) A Saudi infant with vici syndrome: Case report and literature review. Open Access Macedonian Journal of Medical Sciences 6(6): 1081-1084.
© 2023 Stephen F Kralik. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






