- Submissions

Full Text
Techniques in Neurosurgery & Neurology
Brain Metastases, Update in the Management
Gustavo Zomosa R1*, Lucas Gonzalez J2, Gonzalo Miranda G3 and María F Galleguillos E4
1Neurology-Neurosurgery Department, Hospital Clínico Universidad de Chile, Santiago de Chile
2Medical Student Faculty of Medicine, University of Chile, Santiago de Chile
3Imaging Center, Hospital Clínico Universidad de Chile, Santiago de Chile
4Radiology Resident, Hospital Clínico Universidad de Chile, Santiago de Chile
*Corresponding author: Gustavo Zomosa R, Neurology-Neurosurgery Department, Hospital Clínico Universidad de Chile, Santiago de Chile
Submission: December 11, 2018;Published: December 19, 2018

ISSN 2637-7748
Volume2 Issue2
Abstract
Brain metastases represent a critical stage of oncological disease and its frequency has increased over the recent years. The treatment of brain metastases has moved from a conservative approach to an active management that should be individualized for each patient. In cases of single brain metastasis, surgery or radiosurgery should be considered as first option for treatment. In cases of multiple lesions, whole-brain radiotherapy is the standard of care. The aim of this review is to present general aspects including new approaches in the management of patients with brain metastases.
Keywords: Brain metastases; Radiosurgery; Whole brain radiation therapy; Brain surgery; Immunotherapy; Palliative care
Introduction
Brain metastases (BM) are the main direct neurological complication of cancer and are the most frequent intracranial tumors in adults. They are ten times more frequent than primary cerebral neoplasms [1] and its current management involves mostly palliative therapy but is at the moment evolving towards an active therapy such as by surgical means or radiosurgery which has shown a better prognosis. The number of published works on this pathology only amounts to 25% relative to publications on primary brain tumors such as gliomas [2].
Global Epidemiology
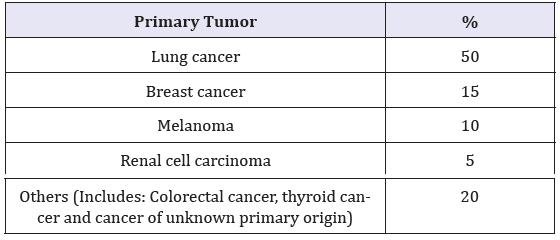
Metastases to the Central Nervous System (CNS) are common, affecting up to 30% of patients with cancer [3]. BM are the most common form of intracranial malignancy with an incidence varying between 8.3 and 14.3 per 100,000 [4]. Mortality rises with age [5]. The list of primary tumors with frequent metastasis to the brain is shown in Table 1, highlighting lung cancer as the prime site of BM origin, as well as breast cancer and melanoma.
Table 1:Origin of primary cancer of brain metastasis [6].
Physiopathology
Several processes are required for metastasis to the CNS to develop from distant loci. This involves malignant cells that escape from the primary tumor, degradation of the extracellular matrix, intravasation into blood vessels, and survival of cells in hematogenous dissemination. This is followed by entry of the cells into the cerebral circulation, extravasation into the brain parenchyma, and finally proliferation and survival of the tumor cells in the cerebral microenvironment. The cells are able to remain in a state of latency for a prolonged period and are able to establish neovascularization processes by induction of vascular endothelial growth factors (VEFG) [7,8]. The hematogenous entry explains the topographic distribution of the metastases; 80% are located in the hemispheres specifically in Middle and Posterior Cerebral Artery territories [9,10] (Table 2). BM are located especially in the gray-white matter junction (GWMJ) due to the change in the caliber of the vessels or their spiral shape that act as a trap for the cells [10]. Another mechanism involved is the sowing of cancer by direct extension to the meninges adjacent to the base of the skull, a common mechanism in osteo-affinity neoplasms such as breast and prostate cancer [12]. One of the endpoints of tumor growth causing mass effect, edema, and obstruction of cerebrospinal flow is intracranial hypertension (IH) which may lead to brain structure herniation and eventual death [13].
Table 2:Brain localization of BM.
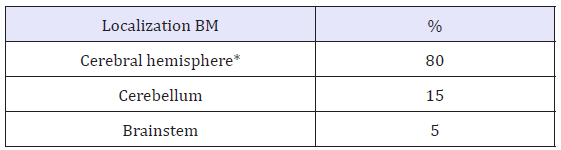
*In order of descending frequencies: frontal lobe, parietal, temporal and occipital [11].
Anatomical Pathology
There are 3 varieties of BM: intraparenchymal tumors, leptomeningeal carcinomatosis, and milliary metastases. Intraparenchymal tumors are the most common variety and may be single or multiple. Macroscopically, the lesions lie within the GWMJ and are superficial, well-circumscribed, surrounded by edema, with margins that compress neighboring tissues. Microscopically, there may be a histologic similarity with the primary tumor or it may be undifferentiated. The majority of cases show considerable anaplasia with foci of hemorrhage, necrosis, and mitosis. In general, they tend to have well-demarcated edges with adjacent reactive brain tissue. However, tumors such as melanomas and small-cell metastatic lung carcinomas tend to infiltrate adjacent tissues. Colorectal adenocarcinomas are associated with extensive necrosis, sometimes with a thin border of viable tumor. Melanoma, renal cell carcinoma, and choriocarcinoma have metastatic tumors that classically cause hemorrhage [14]. Leptomeningeal carcinomatosis is relatively frequent and are present in around 10% of patients with CM [15]. This variety of CM is common in patients with primary cancer of pulmonary origin, lymphomas, leukemias, breast cancer, and melanoma and causes a picture of hydrocephalus, cranial nerve palsy, or infiltration of spinal roots [16]. Milliary metastasis is very rare and will not be discussed in this document.
Clinical Presentation
The development of BM is one of the most devastating complications of tumor progression due to rigidity of the cranial compartment that even small lesions can have clinical effects. BM are symptomatic at some point in the history of the disease in 67% of patients [3]. The symptoms can be focal or generalized, depending on the location. BM are more frequent between the fifth and seventh decades of life. BM are multiple in 75% of patients, and in more than 80% refers to the history of cancer at the time of diagnosis (metachronous presentation), especially lung cancer, breast or melanoma [17] (Table 1). Clinical presentation may be similar to other intracranial tumors such as IH, seizures, pseudodementia and focal syndromes. 20% of patients debut with acute clinical presentation (pseudovascular), particularly melanoma and kidney carcinoma that can bleed easily [18]. Headache as an initial symptom is present in only 50% of patients. Initially, it is oppressive, of slight intensity, and may increase in the following days and weeks, adding the appearance of signs of neurological focality. Subsequently, the headache progresses and develops into “classic” presentation of headache in brain tumors with severe pain, worse in the morning, and associated with nausea and explosive vomiting. There may be isolated episodes of severe headache from 5 to 20 minutes in duration in patients with IH, associated with nausea, emesis, ataxia and obtundation, often triggered by Valsalva maneuver, positional changes, or other movements that cause abrupt elevation in intracranial pressure and loss of regulation of vascular tone. Alterations of consciousness are present in approximately ⅓ of patients, ranging from confusion, drowsiness, or coma. It is common to find papilledema in the fundus. The manifestations of IH may become more evident as the disease progresses. In addition, vomiting can occur with or without nausea, caused by compression on the brainstem, accompanied in some cases with dizziness but not of the vertiginous type. Sudden vomiting without preceding nausea occurs in patients with posterior fossa or hydrocephalus tumors, but this is uncommon [19,20]. Seizures are also frequent [13] and 25% of patients with BM will die from neurological causes [21].
Diagnosis
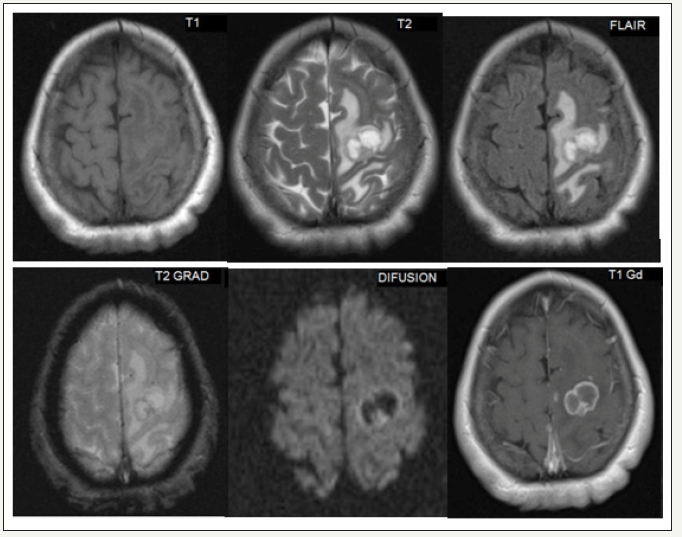
Clinical history, physical examination, and laboratory values guide the diagnosis of BM with the imaging for confirmation. It is recommended as a screening only in patients with small cell lung cancer [21]. Contrast Computed tomography (CT) is the preferred initial diagnostic modality for patients with neurological deficits and suspected metastasis due to its high availability, tolerance, and ability to rapidly diagnose serious pathologic states such as hemorrhage, hydrocephalus, and IH. Although CT is not sensitive enough to detect all metastases, it is still beneficial in diagnosis of BM (Figure 1). The findings that guide the diagnosis of metastasis are iso or hypodense lesions relative to gray matter, usually without calcifications, and with margins of significant perilesional vasogenic edema with intense enhancement after administration of contrast medium. Its appearance is that of tumors located in the GWMJ, with edema in the white substance in a finger-shaped manner in the brain. Magnetic resonance imaging (MRI) with the use of gadolinium is more sensitive for the detection of CM as it detects about 20% more metastases than CT. It is most useful for further characterization of lesions detected on CT, for finding metastasis in patients with high suspicion and negative CT, and for surgical planning [20]. The findings in MRI are usually well circumscribed lesions that are iso- or hypointense in T1 sequences and hyperintense in T2, with intense enhancement after gadolinium administration (Figure 2). Some metastases such as melanoma are hyperintense in T1 sequences due to the paramagnetic effect of melanin, while hemorrhagic metastases may also present with hyperintensity in T1 depending on the temporality of the hemorrhage. Generally, solid areas of the tumor do not restrict diffusion, except for lesions with a hemorrhagic component or high cellularity. They have extensive vasogenic edema disproportionate to the size of the lesion, however, there are some lesions that may not have significant edema [22,23]. Biopsy or surgical resection is indicated to confirm the diagnosis, especially in patients with single lesions without diagnosis of cancer or metastatic disease. The diagnosis of BM can be made with neuroimaging, but if there is uncertainty as to the etiology of the tumor, the histological diagnosis becomes necessary either with biopsy or resection and CSF cytology in special cases [24]. The differential diagnosis includes glioblastoma multiforme which requires many times a directed study with biopsy for the correct diagnosis. Other less frequently occurring differential diagnosis are primary CNS lymphoma and brain abscess that simulate metastatic lesions in the neuroimage.
Figure 1:(CT metastasis, primary lung cancer).
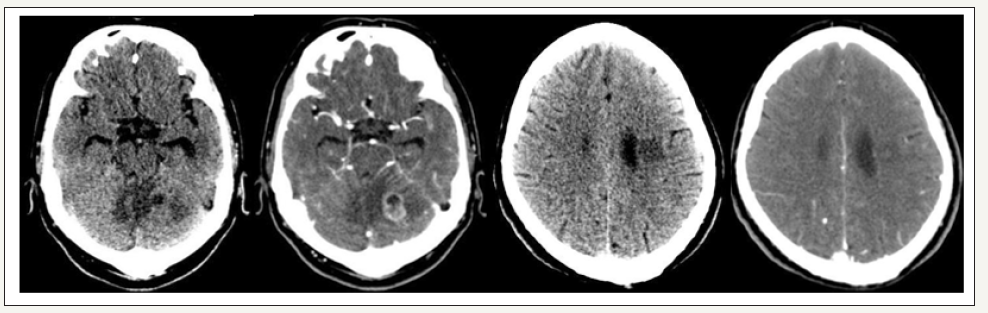
Figure 2:(MRI metastasis, primary lung cancer).
Immunohistochemistry
When there is clinical uncertainty, the immunohistochemical panel (IHC) can help to determine the neoplastic origin that has metastasized to SNC. This can be achieved by joining the information derived from the clinical history, imaging studies and other laboratory tests [25]. In 15% of BM, the origin of the primary tumor is not known, so IHC is useful to determine its source [19]. Neuroepithelial markers should be included in any panel of antibodies when one of the differential diagnoses is a primary CNS neoplasm, however, it is important to consider the expression of neuroepithelial markers in some secondary tumors [26]. Glial fibrillary acidic protein (GFAP) antibodies are useful for metastatic tumors including myoepithelial carcinomas and some neuroendocrine carcinomas. S100 proteins (S-100) are useful for metastatic melanomas [27]. Synaptophysin allows the evaluation of neuroblastomas and neuroendocrine carcinomas. Neural cell adhesion molecule (NCAM or CD56) is useful for the evaluation of leukemias and metastatic lymphomas [26]. Cytokeratins (CK) are fibrous polypeptides that constitute the main type of intermediate filaments in the epithelial cell cytoskeleton, including mucosa and glands, and in some non-epithelial cells [28]. The most used in carcinoma of unknown origin are CK-7 and CK-45 [29], which are low molecular weight CK with anatomical distribution restricted to its epithelium and neoplasms [30]. CK-7 is present in cancers of the lung, ovary, endometrium and breast and it is absent in those of the gastrointestinal tract, prostate and kidney [30-32]. CK-20 is usually expressed in the gastrointestinal epithelium, urothelium and Merkel cells [29]. The combination of these two CKs help in differentiating different tumors [29]. Thyroid Transcription Factor-1 (TTF-1) is a nuclear protein that is essential in morphogenesis and differentiation of thyroid, pulmonary and forebrain structures. It is expressed in the nucleus of pulmonary epithelium, the thyroid, and its tumors and in the CNS [29,33]. Immunohistochemistry is useful to determine the origin of the primary if it is not known. However, the immunohistochemical profiles of metastases can be non-specific and variable. There is no IHQ test that is 100% specific, so the IHQ markers are helpful adjuncts that, together with clinical presentation and radiological studies, will better determine the definitive primary tumor origin [13,29].
Prognostic Factors
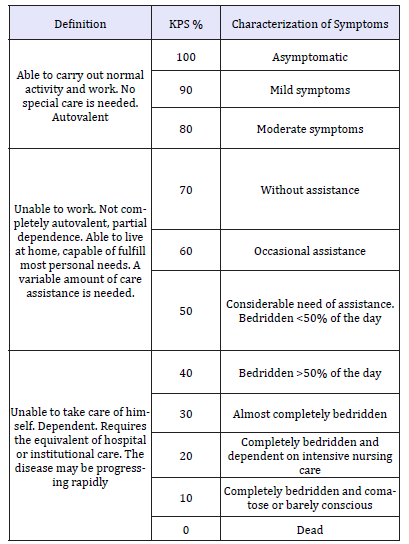
The most important factors that predicts survival of patients with BM are: age of < 65 years, performance status on the Karnofsky scale (KPS) more that 70% (Table 3), control of primary cancer, and absence of extracranial metastases [25]. There are several prognostic scales such as the Repercussive Partitioning Analysis Classification System (RPA) scales and the Graded Prognostic Scoring System (GPA). In this review we will only focus on the RPA scale, that has proposed the classification of BM into 3 groups according to survival [35].
Table 3:Karnofsky Performance Score (KPS) [34].
Initial and Symptomatic Treatment
The average survival without treatment is variable and is estimated to be between 28 and 51 days [20,36]. BM that require urgent treatment for symptomatic improvement and prevent neurological deterioration, are those who present with seizures, altered levels of consciousness, cerebral infarctions, intracranial hemorrhage, or elevation of IP due to its consequences on cerebral perfusion leading to cerebral herniation and eventual death. The initial management should focus on ensuring airway stability, respiration, and circulation (ABC assessment: Airway, Breathing and Circulation) followed by symptomatic management.
Glucocorticoids (GC)
GC reduces vasogenic edema, decreasing the permeability of abnormal tumor capillaries. Dexamethasone is commonly used due to its low mineralocorticoid effect, long half-life, and low tendency to induce psychosis. High doses should be used in patients with severe symptoms, significant mass effect, or those who do not respond to treatment within 48 hours. Patients show clinical improvement hours after the first dose of GC, with a maximum effect after 3-7 days. Common short-term side effects of GC include insomnia, increased appetite, fluid retention and edema, mood symptoms, acne, and exacerbation of diabetes. Chronic side effects include weight gain, steroid myopathy, immunosuppression, and bone necrosis of the femoral head [37].
Anticonvulsants
Patients who develop seizures should be treated with anticonvulsant agents such as levetiracetam, phenytoin sodium, carbamazepine, or valproic acid, with valproic acid as the preferred drug in patients receiving concomitant chemotherapy. The prophylactic use of these is generally not done to patients who have not experienced seizures because primary prevention with anticonvulsants is associated with adverse effects and may not reduce the risk of seizures [38].
Prophylaxis and treatment of venous thromboembolism
Oncology patients have an increased risk of venous thrombosis [36]. However, patients with BM have an increased risk of intracranial hemorrhage if they are given anticoagulation, so their routine use is not recommended, and anticoagulation should be avoided in tumors with propensity to bleed [39].
Active Treatment
The goals of the treatment are to alleviate the neurological symptoms and improve the quality of life (QOL). BM is curative care on rare occasions. At present, the existing therapeutic options are Whole Brain Radiation Therapy (WBRT), surgery, Stereotactic Radiosurgery (SRS), and systemic therapies. In the management of any oncological condition it is important to consider the triad of the patient, the tumor, and the treatment factors in order to choose an appropriate, personalized therapeutic plan. This is to maximize survival and minimize morbidity associated with treatment side effects [40]. We will refer to general aspects of the different existing therapeutic modalities:
Surgery
Surgery allows a rapid reduction of the mass effect and pathological analysis (biopsy) when the diagnosis is not clear. It must be considered if there is a single metastasis, especially when it has an associated extensive cerebral edema and mass effect [41]. It is an effective treatment for unique brain metastases and this intervention improves survival and functionality.
The candidates for surgery [39] are patients with good prognostic factors such as:
A. Controlled primary with an expected survival greater than 6 months
B. Age under 65 years
C. Single BM or when multiple, no more than 2 when they are in the same surgical field and can be resected in a single surgery.
D. Location in non-eloquent area
E. Large volume tumor (> 8 cc)
F. Good functional status (KPS >70%)
G. The primary tumor is unknown and histological diagnosis is required
Whole Brain Radiation Therapy (WBRT)
WBRT is the standard treatment in multiple BM because it decreases neurological symptoms and increases survival [41]. It should be considered in large volume BM outside the scope of SRS, in recurrent BM, or in patients with low KPS (Table 3 & 4). The recommended dose is 20 Gy in 5 fractions or 30 Gy in 10 fractions [42]. The response rate of WBRT is 40-60%, while the rate of improvement or neurological preservation is 25-40%. WBRT is beneficial in patients with high KPS but there are no benefits in patients with low KPS [43]. As the only treatment, it increases the survival from 3 to 6 months. In patients with low functional status, there is no significant difference between good paliative care and WBRT of 20 Gy in terms of survival.
Table 3:Karnofsky Performance Score (KPS) [34].

Table 4:Recursive partitioning analysis (RPA) classification [42].
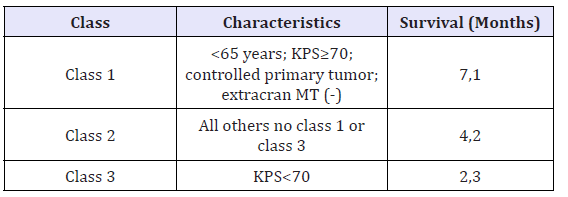
Abbreviations: Extracran MT: extracranial metastases
Acute side effects: Initially, WBRT can worsen cerebral edema. In patients with large tumors and evident mass effect, the GC should be administered 48 hours before the start of radiotherapy with progressive reduction of the dose during therapy. Other shortterm side effects include alopecia, mild skin reaction in the scalp, and fatigue that improves after weeks. Some patients may experience ototoxicity [44].
Late adverse effects:
A. Changes in white matter, neurocognitive impairment, and dementia [45]
B. Ataxia and urinary dysfunction due to normotensive hydrocephalus
C. Neuroendocrine disorders (hypothyroidism)
D. Cerebrovascular disease
E. Radionecrosis [46]
Late adverse effects are dependent on the dose and the fractionation scheme used (current schemes are low risk). The risk of these complications is related to the age of the patient, the degree of the disease, and the level of neurological deterioration in the initial clinical assessment. There are neuroprotective strategies such as radioprotection of temporal hippocampi. Currently, the use of WBRT has been questioned due to the decrease in survival benefits and the risk of severe adverse effects especially in cognitive functioning, with SRS being preferred [39].
Stereotactic Radiosurgery (SRS)
SRS can be very useful for the treatment of a limited number of small tumors that are not surgically accessible [47]. Currently, patients with single or multiple intracranial metastases (< 5) with a size less than 3cm in diameter can undergo SRS as a primary treatment [48]. The risk of neurotoxicity and local failure increases with larger tumors and therefore the use of SRS is limited to lesions less than 3 cm in diameter in a dose between 20 and 25 Gy [49]. There are 2 fundamental types of SRS, the Leksell Gamma knife (LGK) and the linear accelerator (LINAC) with its robotic variant the Cyber Knife [47]. Specially with the last and the new GK Icon there is possible to treat larger lesions by performing hipofractionated SRS to lower toxicity. The reported survival for patients in RPA I is from 18 to 24 months, RPA II from 9 to 11 months and RPA III only from 3 months. Its efficacy is similar with radiosensitive and radioresistant tumors [43]. Recently, the complementary role between SRS and surgery for less than 4 lesions has been proposed, operating one and treating the others with SRS [21]. Also, there are other modalities such as SRS in the post-surgical cavity sometimes difficult to define the proper target because of this it has reported SRS previous to surgical resection. In recent years it has been pointed out the role of SRS as an activator of the immune response specially in melanoma BM after the use of targeted therapies, 14 days with 20 to 25 Gy in a Phase II study.
Systemic Therapies
its inability to cross the BBB. Systemic anticancer agents must reach an adequate concentration in the tumor microenvironment and there are several hurdles to achieve this objective. The BBB has tight junction limiting penetration of large molecules across it, moreover it prevents movement of hydrophilic, ionized and protein bound molecules. Also, endothelial cells exhibit transmembrane efflux pumps that export chemicals [50-52]. Only we are going to discuss about immunotherapy and its role in BM.
Immunotherapy
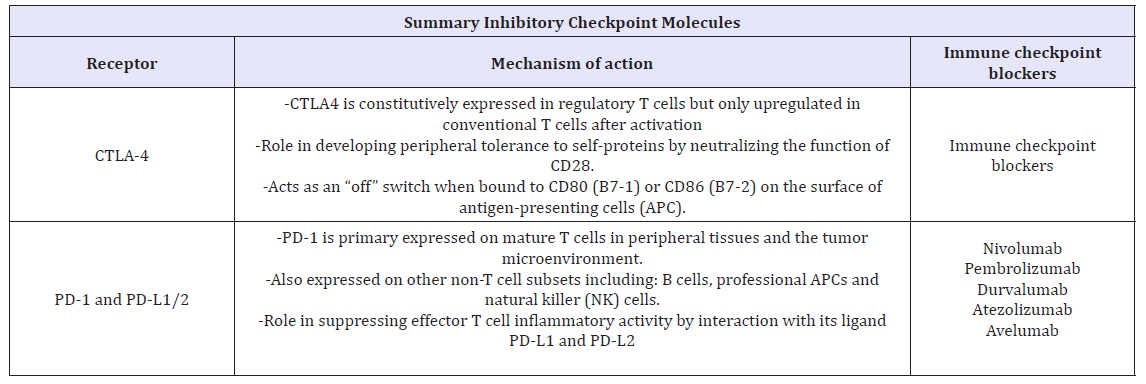
Immune checkpoints are regulators of immune activation. These pathways are crucial for immune homeostasis and self-tolerance (preventing autoimmunity). In the pathogenesis and progression of CNS malignancies there is a dysregulation of immune surveillance and cancer, immune checkpoints mechanisms are often activated to suppress the anti-tumor immune response and evading immune system detection [53] (Table 5 & 6). Activated T cell are primary mediators of immune effector functions and as such, they express multiple co-inhibitory receptors such as cytotoxic T-lymphocyte- associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1). There immune checkpoint molecules have been shown to modulate T cell responses to self-proteins as well as to chronic infections and tumor antigens [54]. The pathways utilized by these checkpoint proteins are unique and non-redundant [55]. This demonstrates the important role of immune checkpoints in regulating immune homeostasis, and provides a rationale for targeting multiple immune checkpoints to enhance anti-tumor immunity.
Table 5:Comparison of surgery and radiosurgery SRS in BM.
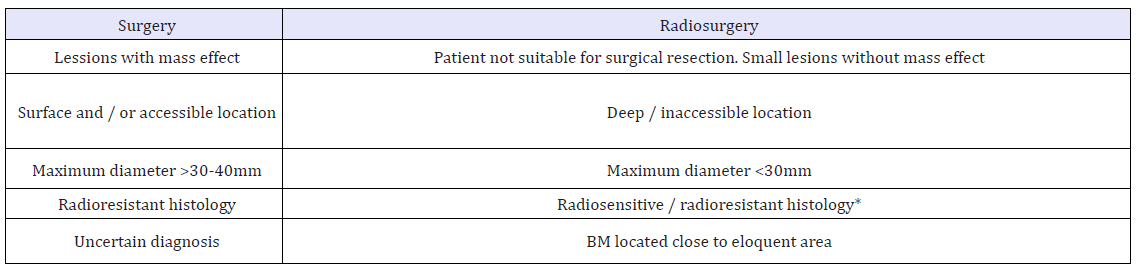
Table 6:
Anti‑CTLA‑4 and/or anti‑PD1 Immunotherapy in Combination With Radiotherapy
RT enhances antitumor immune response, promoting tumor control and there are several evidences about the potential synergic effect of RT with ICB in treatment of BM. RT can trigger the “abscopal effect” which describes treatment response in tumors outside the radiation fields via systemic immune-mediated antitumor effects. This immunostimulatory effect of RT involves the increase translocation and expression of calreticulin to the cell surface and promote gene transcription of proinflammatory factors which are essential part of immunogenic cell death and reduce the production of immunosuppressive cytokines. Also, RT promote re-oxygenation in tumor microenvironment, improving immune cell recruitment. Finally, RT induces the release of antitumor associated antigens and by this, diversifying the T-cell receptor repertoire and increased efficacy of Cytotoxic T lymphocytes.
The combination of RT and ICB has been reported to improve clinical outcomes in multiple metastatic cancers. The clinical evidence supporting the efficacy of combining RT and ICB in treatment of CNS tumors is garnered from melanoma patients with metastatic disease and is retrospective nature. There are several studies suggesting that ipili in combination with RT may be more effective that RT alone in melanoma brain metastases. According to Silk, RT (WBRT or SRS) plus ipili demonstrated and overall survival (OS) benefit of 19,9 months versus 4,0 months for SRS alone, with no associated increase in toxicity with addition of ipili to SRS. Also the timing matters, patients who received SRS before or concurrently with ipili appeared to exhibit improved outcomes in OS and distant intracranial control compared to patients who received RT after immunotherapy [53,56-63].
Conclusion
A. BM are a heterogeneous group of diseases that are prevalent with increasing frequency and of great importance for the general practitioner and specialist
B. The three most common causes of BM are lung cancer, breast cancer and melanoma. In general, they are patients with a known cancer.
C. Highlight the importance of a good clinical history, including anamnesis and physical examination.
D. Use of prognostic functional scales such as KPS, RPA and GPA
E. One of the most important survival factors is the control of primary cancer.
F. The therapeutic decision for each patient must be made individually and meticulously in a multidisciplinary neuro-oncological committee, according to factors of the triad of the patient, the tumor, and available treatments.
G. The prognosis of patients with BM has changed in recent years thanks to progress in diagnosis and cancer management, which has allowed better staging to design the best therapeutic strategies that significantly improve their prognosis and decrease neurologic-associated death.
Table 7:Summary of current management for BM.

Acknowledgement
We thank Jennifer Camile N. Foronda, University of the East Ramon Magsaysay Memorial Medical Center, Inc. for medical writing and editorial assistance.
Author Contribution
LGJ performed the literature research. All authors helped to write the manuscript
References
- Saha A, Ghosh SK, Roy C, Choudhury KB, Chakrabarty B, et al. (2013) Demographic and clinical profile of patients with brain metastases A retrospective study. Asian J Neurosurg 8(3): 157-161.
- Preusser M, Weller M (2015) Brain metastases: A late awakening. Chin Clin Oncol 4(2): 17.
- Barani IJ, Larson DA, Berger MS (2013) Future directions in treatment of brain metastases. Surg Neurol Int 4 (Suppl 4): S220-S330.
- Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14(1): 48-54.
- Ostrom QT, Wright CH, Barnholtz SJS (2018) Brain metastases: Epidemiology. Handb Clin Neurol 149: 27-42.
- Ellison D, Love S, Chimelli L, Harding BN, Lowe JS, et al. (2013) Neuropathology. (3rd edn), Chapter 46, Neoplasms that spread to the CNS. Elsevier, USA, pp. 849-856.
- Sajama C, Lorenzoni J, Tagle P (2008) Diagnósticoy tratamiento de las metástasis encefálicas. Rev méd Chile 136(10): 1321-1326..
- Kienast Y, Von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, et al. (2010) Real time imaging reveals the single steps of brain metastasis formation. Nat Med 16(1): 116-122.
- Delattre JY, Krol G, Thaler HT, Posner JB (1988) Distribution of brain metastases. Arch Neurol 45(7): 741-744.
- Hwang TL, Close TP, Grego JM, Brannon WL, Gonzales F (1996) Predilection of brain metastasis in gray and white matter junction and vascular border zones. Cancer 77(8): 1551-1555.
- Yachnis A, Rivera Zengotita M (2014) Metastatic Brain Tumors. In: Neuropathology, (1st edn), Elsevier, USA, pp. 191-199.
- Nonaka H, Akima M, Hatori T, Nagayama T, Zhang Z, et al. (2003) The microvasculature of the cerebral white matter: arteries of the subcortical white matter. J Neuropathol Exp Neurol 62(2): 154-161.
- Lassman AB, DeAngelis LM (2003) Brain Metastases. Neurol Clin 21(1): 1-23.
- Cereceda L (2011) Oncologic emergencies. Rev méd Clín. Las Condes 22(5): 545-695.
- Klos KJ, O Neill BP (2004) Brain metastases Neurologist 10(1): 31-46.
- Chamberlain M, Junck L, Brandsma D, Soffietti R, et al. (2017) Leptomeningeal metastases a RANO proposal for response criteria. Neuro Oncol 19(4): 484-492.
- Rees J, Wen PY (2010) Neuro-Oncology. (1st edn), Elsevier, USA.
- Hofer S, Brada M (2010) 13-Brain Metastases. Elsevier, USA, pp. 284- 296.
- Barnholtz SJS, Sloan AE, Davis FG, Vigneau FD, Lai P, et al. (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol 22(14): 2865-2872.
- Bernstein M, Berger MS (2014) Neuro oncology. The Essentials (3rd edn), Thieme Germany.
- Patel AJ, Lang F, Sawaya R. Metastatic Brain Tumors. Thieme Germany pp. 451-461.
- Forsyth PA, Posner JB (1993) Headaches in patients with brain tumors a study of 111 patients Neurology 43(9): 1678-1683.
- Mezzadri JJ, Goland J, Socolovsky M (2011) Introducción a la neurocirugía. (2nd edn), Ediciones.
- Journal Socolovsky M (2011) Tumores neuroepiteliales, metástasis y meningiomas. Ediciones Journal pp. 121-39.
- Luján M (2005) Diagnostic evaluation and basic management of brain metastases. Rev Colomb Cancer 10(1): 61-66.
- Batchelor T, Nishikawa R, Tarbell N, Weller M (2017) Oxford Textbook of Neuro-Oncology (1st edn), Oxford University Press pp. 272.
- Preusser M, Schackert G, Baumert BG Chapter 19 Metastatic brain tumours. Oxford University Press, UK, pp. 520-546
- Fink K, Fink J (2013) Imaging of brain metastases. Surg Neurol Int 4(5): 209-219.
- Osborn AG (2013) Osborn’s Brain Imaging, Pathology, and Anatomy Edition (1st edn), Amirsys, Salt Lake City, Utah, USA, pp. 745-772.
- Greenberg MS (2016) Handbook of neurosurgery Edition. (8th edn), Thieme Chapter 52, Cerebral Metastases Thieme, pp: 800-813.
- Niederhuber JE, Armitage JO, Doroshow JH, Kastan MB, Tepper JE (2014) Abeloff’s Clinical Oncology. (5th edn), Elsevier, USA, P. 2186.
- Sneed PK, Kased N, Rubenstein JL (2014) Chapter 50 Brain Metastases and Neoplastic Meningitis. Elsevier 725-738.
- Bouvier C, Fernandez C, Meyronet D, Figarella Branger D (2005) Examens cytologique, histologique, immunohistochimique et génétique des tumeurs du système nerveux central EMC-Neurol 2(4): 557-585.
- Péus D, Newcomb N, Hofer S (2013) Appraisal of the karnofsky performance status and proposal of a simple algorithmic system for its evaluation. BMC Med Inform Decis Mak 13(1): 72.
- Rebolledo V, Colombo C, Rebolledo L (2016) Expresión de citoqueratinas en el cáncer de mama y subtipos tumorales por inmunohistoquímica Rev Obstet Ginecol Venez 76(2): 93-101.
- Sánchez de Ibargüen CB, Sánchez Ruiz A, Alonso MC, Hurtado Nuño A, et al. (2006) Carcinoma de origen desconocido: diagnóstico y manejo terapéutico. Oncol 29(3): 95-106.
- Chu P, Wu E, Weiss LM (2000) Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: A survey of 435 cases. Mod Pathol 13(9): 962-972.
- Ramaekers F, Huysmans A, Schaart G, Moesker O, Vooijs P (1987) Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res 170(1): 235-249.
- Rubin BP, Skarin AT, Pisick E, Rizk M, Salgia R (2001) Use of cytokeratins 7 and 20 in determining the origin of metastatic carcinoma of unknown primary, with special emphasis on lung cancer. Eur J Cancer Prev 10(1): 77-82.
- Boggaram V (2009) Thyroid transcription factor-1 (TTF-1/Nkx2.1/ TITF1) gene regulation in the lung. Clin Sci 116(1): 27-35.
- Langley RE, Stephens RJ, Nankivell M, Pugh C, Moore B, Navani N, et al. (2013) Interim data from the Medical Research Council QUARTZ Trial: Does whole brain radiotherapy affect the survival and quality of life of patients with brain metastases from non-small cell lung cancer? Clin Oncol 25(3): e23-e30.
- Lin X, DeAngelis LM (2015) Treatment of brain metastases. J Clin Oncol 33(30): 3475-3484.
- Gaspar L, Scott C, Rotman M (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastasis trials. Int J Radiat Oncol Biol Phys 37(4): 745- 751.
- Lobos Urbina D, Kittsteiner Manubens L, Peña J (2017) Is primary prevention with antiepileptic drugs effective in brain tumors or brain metastases? Medwave 17(Suppl1): e6871.
- Hunter BD, Minichiello T, Bent S (2017) Anticoagulation for the treatment of venous thromboembolism in patients with brain metastases: a metaanalysis and systematic review. J Thromb Thrombolysis 44(3): 392-398.
- Hardesty DA, Nakaji P (2016) The current and future treatment of brain metastases. Front Surg 3: 30.
- Rabadán AT, Diez B, Martínez AM, Antico J, Saidón P, Christiansen S, et al. (2006) Consenso para el tratamiento de las metástasis cerebrales. Rev argent neurocir 20(4): 179-193.
- Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases. JAMA 295(21): 2483-2491.
- Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, et al. (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet 363(9422): 1665-1672.
- Pease NJ, Edwards A, Moss LJ (2005) Effectiveness of whole brain radiotherapy in the treatment of brain metastases: A systematic review. Palliat Med 19(4): 288-299.
- Loeffler JS, Wen PY (2018) Overview of the treatment of brain metastases. UpToDate.
- DeAngelis LM, Delattre JY, Posner JB (1989) Radiation-induced dementia in patients cured of brain metastases. Neurology 39(6): 789-796.
- Walker AJ, Ruzevick J, Malayeri AA, Rigamonti D, Lim M, Redmond KJ, et al. (2014) Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Futur Oncol 10(7): 1277-1297.
- Smith ML, Lee JY (2007) Stereotactic radiosurgery in the management of brain metastasis. Neurosurg Focus 22(3): E5.
- Badiyan SN, Regine WF, Mehta M (2016) Stereotactic radiosurgery for treatment of brain metastases. J Oncol Pract 12(8): 703-712.
- Owonikoko TK, Arbiser J, Zelnak A, Shu HG, Shim H, et al. (2014) Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol 11(4): 203-222.
- Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, et al. (2011) The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol 8(6): 344-356.
- Fortin D (2012) The blood-brain barrier: Its influence in the treatment of brain tumors metastases. Curr Cancer Drug Targets 12(3): 247-259.
- Venur VA, Karivedu V, Ahluwalia MS (2018) Systemic therapy for brain metastases. Handb Clin Neurol 149: 137-153.
- D Souza NM, Fang P, Logan J, Yang J, Jiang W, et al. (2016) Combining radiation therapy with immune checkpoint blockade for central nervous system malignancies. Front Oncol 6: 212.
- Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4): 252-264.
- Nirschl CJ, Drake CG (2013) Molecular pathways: Coexpression of immune checkpoint molecules: Signaling pathways and implications for cancer immunotherapy. Clin Cancer Res 19(18): 4917-4924.
- Caponnetto S, Draghi A, Borch TH, Nuti M, Cortesi E, et al. (2018) Cancer immunotherapy in patients with brain metastases. Cancer Immunol Immunother 67(5): 703-711.
- Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD (2013) Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2(6): 899-906.
© 2018 Gustavo Zomosa R. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






