- Submissions

Full Text
Surgical Medicine Open Access Journal
Pregnancy Associated Acute Pancreatitis: A Practical Approach
Zafar Chawdhery M1, Farkhanda Dahri2, Shahab Uddin3 and Kulsoom Junejo4*
1Consultant General Surgeon, UK
2People’s University Medical Health Science Nawabshah, Pakistan
3Translational Research Institute and Dermatology Institute, Qatar
4Department of Surgery, Qatar
*Corresponding author:Kulsoom Junejo, Department of Surgery, Hamad General Hospital, Doha, Qatar
Submission: November 22, 2022Published: August 07, 2023

ISSN 2578-0379 Volume5 Issue3
Abstract
Pregnancy associated with acute pancreatitis is a potentially life-threatening but rare event occurring in approximately three in 10000 pregnancies. Its prompt diagnosis warrants a high degree of suspicion in women who present with severe upper abdominal pain which may or may not radiate to the back. These patients usually have accompanying anorexia, nausea and vomiting. Serum amylase, lipase, CRP and abdominal ultrasound should be included in the initial workup for these symptoms occurring during pregnancy. It occurs usually during the third trimester or the early postpartum period. Once the diagnosis of acute pancreatitis is established, the mainstay of treatment is symptomatic including aggressive fluid resuscitation if not otherwise contraindicated and pain relief; antibiotics should not be initiated at this stage. Next it would be imperative to ascertain the severity of the disease using established criteria such as the modified Glasgow or Atlanta’s criteria within the first 48 hours of admission.
The patient is kept under close observation with fetal monitoring and swift action taken on signs of clinical deterioration e.g., rising respiratory rate, worsening pain, increasing tachycardia, hypoxia or low urinary output. Most pregnant women with moderate acute pancreatitis and all with severe disease will require intensive care support. Gallstones, hypertriglyceridemia and alcohol use are the main causes which will necessitate treatment consideration on an individual basis. Mild disease usually resolves without any major intervention or sequel whereas timing of intervention in others is crucial for good outcome. To minimize maternal and fetal mortality and morbidity diligent decision-making related to the termination of pregnancy and the management of acute pancreatitis with its underlying pathology will require a multidisciplinary team approach. This should include obstetrician, surgeon, interventional gastroenterologist, radiologist and ICU staff.Keywords:Pregnancy; Acute pancreatitis; Management; Outcome; Fetal loss
Introduction
Gestational acute pancreatitis being sudden onset of pancreatic inflammation is an uncommon occurrence affecting approximately three in 10,000 pregnant women during the third trimester or the early postpartum period. Its incidence ranges from 1 in 1,000-10,000 depending on the diagnostic criteria used and geolocation as accurate diagnosis is not always achieved [1-3]. Maternal mortality due to Pregnancy Associated Acute Pancreatitis (PAAP) was earlier reported to be at 37% and fetal death rate at 60% [4,5]. However, PAAP related fetal mortality in one report in 2014 appeared to have dropped significantly to about 3% and maternal death rate from PAP has declined to 0% [6]. This reduction probably is due to enhanced awareness, more advanced diagnostic modalities and therapeutic manipulations available now a days. The disease occurs most frequently during the third trimester and is associated with a higher frequency of preterm deliveries or in early postpartum period [7].
The causal distribution is somewhat dissimilar to that of the non-pregnant population and diagnostic challenges in pregnancy potentially substantial. PAAP may occur on the background of preformed or de novo gallstone disease, hypertriglyceridemia or alcohol abuse whereas in some cases no causation may be found [8]. Hypertriglyceridemia (HTG) per se appears to exacerbate the severity score and prognosis of PAAP [3,7] and there appears to be a link between degree of HTG and severity of pancreatitis [9].
Etiology
Gallstones
Acute pancreatitis develops in less than 10% of patients in the general population with gallstones whereas cholelithiasis is the commonest cause (65% of cases) of acute pancreatitis during pregnancy [10]. Both obesity and multiparity are known risk factors for gallstone formation and this predisposition is likely to be compounded by inherent weight gained during pregnancy. Gallstones are more common in pregnant compared with nonpregnant patients. Complications of gallstones include acute cholecystitis, choledocholithiasis, cholangitis and gallstone pancreatitis. There are increased levels of estrogen and progesterone production during pregnancy influencing physiological changes in the quality of bile and biliary system [11], For example, intrahepatic cholestasis of pregnancy is known to occur in the second or third trimester [12]. It is postulated that pregnancy induced hormonal changes lead to the formation of lithogenic bile, biliary sludge and gallstones. Following the subsequent physiological contraction of gallbladder these microlithiases are pushed down into the bile duct system. This can cause obstruction to the ampulla of Vater leading to outflow obstruction of pancreatic duct raising intraductal pressure and acinar cell discharge within the pancreatic parenchyma and resultant pancreatitis Table 1.
Table 1: Etiology of pregnancy associated acute pancreatitis.
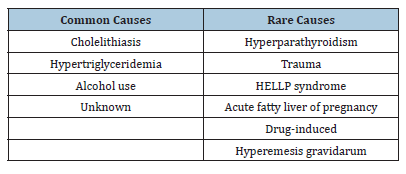
Alcohol
Alcohol use is a major cause of acute pancreatitis accounting for more than 35% of cases in the general population. However, it is uncertain why some alcoholics are more susceptible to developing acute pancreatitis than others who ingest similar quantities of alcohol [13]. It is said that ethanol increases the permeability of pancreatic ductules which leads to digestive enzymes entering the parenchyma causing pancreatic damage at the ductal level whereas at the cellular level pancreatic enzymes accumulate intracellularly. In addition, increase in the protein content of pancreatic juice and decrease in bicarbonate levels and trypsin inhibitor concentrations caused by ethanol leads to the formation of protein plugs within pancreatic ducts that blocks its outflow. This causes release of pancreatic enzymes and their premature activation that results in acute pancreatitis.
Hypertriglyceridemia
Another proposed mechanism for acute pancreatitis in pregnancy in certain women is hormonal dependent high triglycerides levels in the blood during pregnancy. When resultant hypertriglyceridemia ensues, hyperviscous blood will likely cause deformity of red blood cells leading to lower oxygen carrying capacity. Pancreas, being a low blood flow organ in resting state, may not have adequate perfusion, and consequent relative ischemia may predispose to acute pancreatitis.
Uncommon causes
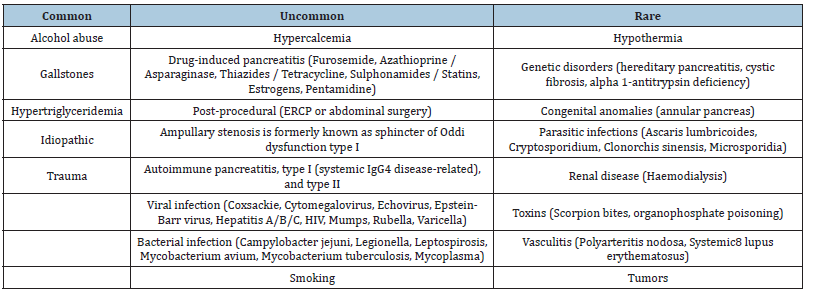
The other reasons for acute pancreatitis as in general population include trauma to the pancreatic ducts, hyperparathyroidism associated hypercalcemia, HELP syndrome (hemolysis, elevated liver enzymes, and low platelet count), some drugs like thiazide diuretics, hyperemesis gravidarum, certain gene mutations (most frequently found SPINK1 mutation is N34S, predominantly found in patients with idiopathic chronic pancreatitis) or pregnancy associated acute fatty liver [5,14-17] Table 2. Recent demographic data from Taiwan suggests that pre-eclampsia alone increases the risk of acute pancreatitis by 1.68-fold [18].
Table 2: Causes of pancreatitis in general population.
Pathophysiology
The underlying cause once triggered the premature and exaggerated activation of the digestive enzymes within the parenchyma leads to pancreatic acinar cell damage and inflammatory response. Alteration in the pancreatic microperfusion happens in the early course of acute pancreatitis regardless of the underlying [19]. As a response to pancreatic injury acinar, stellate cells and other resident immune cells secrete proinflammatory cytokines such as IL-1b, IL-6 and TNF-α. This leads to reduction in blood flow, capillary leakage, pancreatic and peripancreatic oedema and transmigration of inflammatory cells. Other proinflammatory mediators leading to these events are under investigation [20].
Ultimately the decreased microperfusion and activation of the endothelium will also lead to hypercoagulability which, in turn, aggravates pancreatic hypoperfusion and hypoxia. Hypoxia and low reperfusion damage will lead to pancreatic necrosis with sometimes catastrophic consequences such as infected pancreatic collections, sepsis, bleeding and death. Systemically multiorgan failure due to systemic inflammatory response syndrome, hypoperfusion and shock are common events in severe forms of acute pancreatitis, leading to mortality rates close to 50% in some patient cohorts [21].
This results in an increase in vascular permeability and significant fluid shifts from intravascular to peritoneum and third space. Increased vascular endothelial permeability also results in retroperitoneal hemorrhage. Pancreatic enzymes discharged into the systemic circulation can lead to autodigestion of fats causing fat necrosis and release of free fatty acids which react with ionized serum calcium resulting in hypocalcemia [22,23].
Clinical Presentation
A high degree of suspicion of acute pancreatitis is required if the diagnosis is not to be missed. Patients usually present with sudden onset of sharp epigastric pain which classically radiates through to the back. Anorexia, nausea and vomiting may also be present. Clinical examination may be more challenging in the presence of pregnancy, especially in the third trimester. However, tachycardia, fever, signs of peritonitis, epigastric tenderness and absent or reduced bowel sounds may be elicited. Severe necrotizing pancreatitis carries a substantially poor prognosis and may be suspected clinically if Cullen’s or Grey-Turner’s signs are found. Cullen’s sign is a bluish discoloration in the periumbilical area due to hemoperitoneum, and Grey-Turner’s sign is a reddish-brown discoloration, or a ruddy erythema seen in the flanks caused by the retroperitoneal hemorrhage mixed with extravasated pancreatic exudate dissecting through tissue planes.
Laboratory Investigations
There appears to be no specific test for reliably diagnosing all
acute pancreatitis promptly but when it is suspected clinically the
revised Atlanta classification [24] requires that two or more of the
following criteria be present for accurate diagnosis:
A. Upper abdominal pain suggestive of pancreatitis
B. Elevated serum amylase or lipase level greater than three
times the upper normal limit
C. characteristic findings of pancreatitis on imaging.
Serum lipase and amylase levels, when raised more than three times normal are diagnostic but do not directly correlate with severity of the disease. Lipase is relatively more specific than amylase and stays elevated longer than amylase due to its longer half-life in serum resulting from renal tubular reabsorption. Neither have any prognostic significance [25] and their serial estimation should not be performed routinely as these do not correlate with either progression of the disease or its severity.
Complete blood count, Liver enzymes estimation to assess for any concurrent cholestatic element as patients with acute pancreatitis who have an Alanine Transaminase (ALT) level >150U/L has a positive predictive value of 85% for gallstones. Other laboratory tests include renal function test, LDH and blood glucose. Arterial blood gases should also be obtained within 48 hours of admission. Significantly raised C-reactive protein levels after 48 is predictive for the development of pancreatic necrosis [26].
Diagnostic Imaging
Ultrasound is safe during pregnancy and is the imaging technique of choice in pregnant patients. It can identify gallstones, exclude acute cholecystitis, distinguish a normal appearing pancreas from one that is edematous and enlarged. However, bile duct stones may be overlooked on abdomen ultrasound. Endoscopic Ultrasound (EUS) and Magnetic Resonance Cholangiopancreatography (MRCP) are non-invasive imaging studies and do not use ionizing radiation, are helpful in accurately diagnosing a biliary cause for PAAP. Computed Tomography (CT) scan has a major disadvantage due to potential radiation risk to the fetus and must be used with caution if indicated. MRI without gadolinium contrast is considered safe in pregnancy [27].
If facilities for MRCP and EUS are available diagnostic Endoscopic Retrograde Cholangiopancreatography (ERCP) should not be performed due to the associated risks including bleeding, perforation, pancreatitis, fetal radiation. However, ERCP in pregnancy with fetal shielding using lead apron and limiting fluoroscopy exposure to less than 1 min has been reported in a number of studies and case reports where it was used to detect choledocholithiasis [28-30]. Prophylactic antibiotics should be administered to patients who undergo ERCP. The second trimester is probably the optimal time for the use of ERCP when possible teratogenic effects of radiation would be avoided.
Diagnostic Dilemmas in Pregnancy
The diagnosis of PAAP may not be obvious as presenting symptoms may resemble the onset of labor or other causes of acute abdomen like appendicitis, peptic ulcer, pyelonephritis, myocardial infarction, cholecystitis, acute mesenteric ischemia, gastrointestinal or even pancreatic cancer etc. Onset of labor may be triggered by the peritoneal irritation initiated by pancreatic exudate. Inherent complications of pregnancy such acute fatty liver, preeclampsia, HELLP syndrome, placenta abruptio or uterine rupture etc. must also be excluded early on [31]. Abdominal pain in patients in later stages of pregnancy may be due to physiologic changes in pain perception or related to the enlarged uterus displacing maternal organs upward and laterally [32].
Moreover, acute peri-pancreatic collection of fluid in near term patients in the presence of locally complicated disease may render it difficult to arrive at the correct diagnosis [33]. However, in PAAP due to HTG characteristically high serum TG levels (≥1000mg/dL) would be present. Notwithstanding this TG levels usually fall after a few days of fasting and elevated HTG may be concealed as a causative factor. There are incidents of PAAP in patients with TG levels much lower than 1000mg/dL, but HTG was the only identifiable trigger in PAAP [34]. Serum amylase can also be somewhat elevated due to other causes of acute abdomen such as bowel perforation, ectopic pregnancy, or diabetic ketoacidosis. Paradoxically its levels at admission may be within the normal range which can mislead the clinician and diagnosis of PAAP overlooked or delayed [35].
Severity of pancreatitis risk scoring
Acute pancreatitis is categorized as mild, interstitial edematous, or severe necrotizing type pancreatitis. The severity of acute pancreatitis should be assessed within the first 48 hours of admission e.g., by using either Atlanta criteria for severity of pancreatitis Table 3 or modified Glasgow criteria Table 4. Any patient who scores three or more points is likely to have severe pancreatitis and care should be provided in an intensive care setting. Further, other validated risk stratification scoring systems include the APACHE II score, the Ranson Criteria, and Balthazar CT score can also be used for scoring severity of acute pancreatitis [5]. These criteria have prognostic and predictive values and correlate well with the outcome of the disease [36].
Table 3:Modified glasgow score.
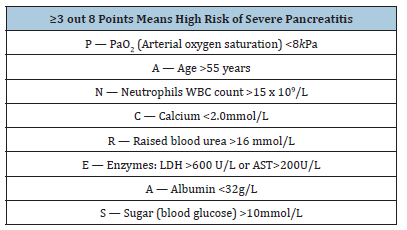
Table 4:Atlanta criteria for severity of pancreatitis.

Management of acute pancreatitis in pregnancy
The treatment of acute pancreatitis in pregnancy is generally similar to that of non-pregnant patients, for example resting the digestive tract, pain control and aggressive fluids given through an IV line [37]. The mainstay of treatment is supportive therapy initially and any subsequent complications that arise can be dealt with later. Adequate Analgesia, fluid replacement and nutritional support are the main tenets and discussed below.
Medical Treatment
Table 5:Pharmacotherapy in pregnancy associated pancreatitis.
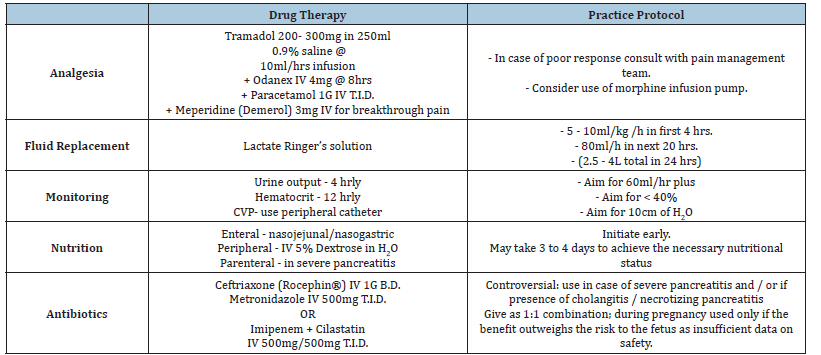
At present there are no medications that are used specifically to treat acute pancreatitis. The main aim of medical therapy is to relieve pain and minimize potential complications. Drug therapy is primarily supportive in nature and involves administration of intravenous fluids to prevent fetal and maternal dehydration, analgesics, antibiotics when indicated and especially in severe acute pancreatitis, and manage any intercurrent metabolic complications such as hypocalcemia or hyperglycemia Table 5. Prophylaxis against venous thromboembolism is mandatory and should be offered using either low molecular weight heparin (Dalteparin/ Enoxaparin), or unfractionated heparin guided by local protocols.
Pain control
It is essential for quality patient care that pain is alleviated to help healing and overcome intercurrent anxiety. Prompt pain relief also promotes patient comfort and prevents pulmonary complications such as basal atelectasis. Paracetamol is peripherally acting drug of choice for mild to moderate pain and mild pyrexia. Tramadol on the other hand is a centrally acting analgesic for moderate to severe pain. It alters perception of and response to pain by inhibiting the ascending pain pathways along with inhibition of norepinephrine and serotonin reuptake. Meperidine (Demerol) on the other hand is a synthetic opioid narcotic analgesia used mainly for the relief of severe pain. Its actions are somewhat similar to morphine. However, it may cause less smooth muscle spasm whereas cough reflex depression is somewhat similar to the analgesic doses of morphine. Constipation appears to be lesser of a problem with Meperidine. My previous favorite propoxyphene, an opioid analgesic used to treat mild to moderate pain, was withdrawn from the US market about ten years ago due to causation of QT prolongation at therapeutic doses.
Fluid resuscitation
An early event in the course of acute pancreatitis regardless of the underlying cause is changes in the pancreatic micro-perfusion [19]. As a response to pancreatic injury acinar, stellate cells and other resident immune cells secrete proinflammatory cytokines such as IL-1b, IL-6 and TNF-α. This leads to reduction in blood flow, capillary leakage, pancreatic and peripancreatic oedema and transmigration of inflammatory cells. Other proinflammatory mediators leading to these events are under investigation [20]. Ultimately the decreased microperfusion and activation of the endothelium will also lead to hypercoagulability which, in turn, aggravates pancreatic hypoperfusion and hypoxia.
The lack of oxygen as well as reperfusion damage will lead to pancreatic necrosis with sometimes catastrophic consequences such as infected pancreatic collections, sepsis, bleeding and death. Systemically multiorgan failure due to systemic inflammatory response syndrome, hypoperfusion and shock are common events in severe forms of acute pancreatitis, leading to mortality rates close to 50% in some patient cohorts [21]. Secondary organ failure due apparent hypovolemia resulting from fluid sequestration can be prevented with aggressive fluid therapy [38].
In the early phase of acute pancreatitis patients can lose a large amount of fluid to the third space, retroperitoneum and intraabdominal areas. Therefore, aggressive fluid resuscitation with both crystalloids and colloids is critically important in the first 24 hours of the diagnosis with prompt Intravenous (IV) hydration. Achieving hemodynamic stability at this stage is critical to prevent organ failures by administration of large quantity of fluid, initially as a bolus followed by continuous infusion at a rate of 250-500ml/h. To ensure adequate hydration central venous pressure, pulmonary artery wedge pressure, and urine output (>0.5-1.0ml/kg/h) should be closely monitored and contemplation of decompensating signs such as hypoxia due to pulmonary oedema and abdominal compartment syndrome resulting from overhydration [39].
Nutritional support of patients with acute pancreatitis
Generally, in patients with mild uncomplicated pancreatitis the caloric intake with IV administration of 5% dextrose in water should be sufficient and no advantage has been observed from additional nutritional support. It is recommended once the patient’s pain and anorexia resolves oral feedings may commence. On the other hand, patients with moderate to severe pancreatitis should receive nutritional support early in the course of the disease and certainly as soon as their condition is stabilized with fluid resuscitation and hemodynamic parameters allow it. If the diagnosis of severe pancreatitis is made on admission nasojejunal feedings with a low-fat formulation may be initiated. A feeding jejunostomy may be placed at the time of any surgery that may be required for diagnosis or treatment of complications of the disease. For enteral feeding there appears to be no advantage of nasojejunal tubes over nasogastric tubes.
Oral feedings low in fat and protein may commence once abdominal pain subsides and the patient feels hungry. In mild acute pancreatitis initiating oral feeding with a low-fat solid diet can be as well tolerated as initiating feeding with a clear liquid diet but it does not necessarily result in a shorter length of hospital stay, as was demonstrated in a prospective, randomized study of 121 patients [40]. There was no difference in rate of death or major infections when in a multicenter study of 208 patients with acute pancreatitis at high risk for complications, were randomized to early nasoenteric tube feeding or an oral diet and if oral diet was not tolerated tube feeding was provided [41]. Total Parenteral Nutrition (TPN) needs to be initiated whenever patient’s required caloric needs cannot be met by the enteral nutrition or there is a failure to maintain adequate jejunal access. However, TPN does not appear to reduce mortality and can lead to significant morbidity e.g., central line infections and metabolic complications like electrolyte imbalance and hyperglycemia.
Moreover, it has its own unique set of impediments causing mucosal atrophy, decreased intestinal blood flow, increased risk of bacterial overgrowth in the small intestine, antegrade colonization with colonic bacteria and increased bacterial translocation. Therefore, whenever feasible enteral nutrition should be preferred over TPN and later should be reserved as a second-line therapy for patients unable to tolerate enteral feeding. Further, it is suggested that the TPN solution should include fat emulsions in sufficient amounts to prevent essential fatty acid deficiency
Antibiotic Therapy
Prophylactic use of antibiotics in severe pancreatitis is controversial. Initial low-grade fever typically does not reflect an infectious process but more likely to be due to Systemic Inflammatory Response Syndrome (SIRS) which is an exaggerated immune response to the stress of inflammation of the pancreas and peritoneum that is attempting to localize and later eliminate the endogenous source of the injury.
Antibiotics are therefore not used routinely on admission but are reserved to combat microorganisms that may grow in biliary pancreatitis or acute necrotizing pancreatitis. The principle of antibiotic use is based on the concept that aerobic gram-bacilli and gut anaerobic microorganisms are the usual culprits in instigating pancreatic infections. However, the antibiotic regimen can later be amended according to actual culture sensitivities obtained.
A combination of intravenous ciprofloxacin and metronidazole failed to confer any advantage in preventing infectious complications of acute pancreatitis in a randomized trial and therefore is not recommended for routine use as prophylaxis in PAAP [42]. However, broad spectrum empirical therapy using intravenous ceftriaxone (Rocephin®) and Metronidazole appears a safe initial treatment while awaiting microorganism culture and sensitivity data.
Alternatively, aImipenem-cilastatin administered in a 1:1 ratio is a useful combination in suspected multi-organism infections requiring broader coverage as shown in a prospective, randomized, multicenter study. Nonetheless, there is insufficient data on safety these drugs during pregnancy and these should be used with caution in PAAP especially if the benefit outweighs the risk to the fetus [43].
Specific Treatment
As stated earlier, gallstones are the commonest cause of PAAP and may be treated by laparoscopic cholecystectomy. It is advised that surgery should be avoided in the first trimester of pregnancy and whenever possible postponed to the second trimester when it may be safely undertaken. However, the decision for cholecystectomy in the third trimester is individualized; it may be offered to the patients during admission or deferred until after delivery of the child.
Managing choledocholithiasis or thick biliary sludge often requires stenting the common bile duct, which may be left in situ until the time of cholecystectomy. However, if waiting until the end of pregnancy is not possible, laparoscopic cholecystectomy can generally be performed safely from the second trimester onwards. Endoscopic therapeutic biliary intervention in the way of sphincterotomy and or stent placement for biliary duct stones using ERCP does require real-time exposure to X-rays and significant amount of fluoroscopic radiation. This potentially is a cause of developmental abnormalities for the fetus and a genuine concern for the clinicians limiting its use in the past.
However, in a recent meta-analysis of 27 studies involving 1,307 pregnant women who underwent ERCP showed that radiation-free techniques are feasible and can significantly reduce the rate of nonpregnancy-related complications. The analysis further revealed that fetal and pregnancy-related complications rates were unaffected [44]. ERCP may be considered a reasonably safe and effective therapeutic procedure during pregnancy when it is performed by experienced gastroenterologists in highvolume centers employing fetal shielding and limited short burst fluorescence exposure [45]. In any event, it should be reserved for patients with persistent bile duct obstruction demonstrated by other imaging modalities, such as MRCP or EUS or those who develop cholangitis [46].
Hypertriglyceridemia associated with acute pancreatitis may be managed effectively by pharmacotherapy and dietary modifications that would help prevent future recurrent attacks. As the end of pregnancy is usually associated with an immediate fall in the triglyceride level, if pancreatitis occurs late in the third trimester, delivery of the fetus is usually advocated. Severe gestational hypertriglyceridemia at full term may also successfully be treated with postpartum therapeutic plasma exchange [47].
The treatment of alcohol associated pancreatitis in pregnancy is the same as in non-pregnant women, starting with preventative Severe acute pancreatitis is associated with significant morbidity and mortality even in this younger population. It should be treated by dedicated experienced clinicians. The mainstay of its management is early endoscopic sphincterotomy and delayed cholecystectomy. However, complications arise as the disease processes progresses further and surgical intervention may be required on individual basis. For example, pancreatic pseudocysts may need cystogastrostomy, pancreatic abscesses may be treated by percutaneous drainage and walled off infected necrosis will necessitate pancreatic necrosectomy in a specialist centre.
Causes of Fetal Loss
The sinister signs of patients worsening conditions such as low urinary output, rising respiratory rate, worsening pain, increasing tachycardia, hypoxia carry poor prognosis unless promptly managed. If despite 24 to 48 hours of active treatment of moderate and severe pancreatitis there is severe fetal distress, fetal malformation, stillbirth, or maternal paralytic ileus termination of pregnancy may well have to considered [48]. In a Chinese retrospective analysis of 54 case cases of PAAP, 57% women delivered at term, 22% had preterm termination of pregnancy. Although no maternal death occurred there was 20% fetal loss. Hyperlipidaemia appeared to have a stronger association with intrauterine fetal distress when compared with other causes of pancreatitis (P<0.01). The incidence of fetal distress and fetal loss were proportional to severity of pancreatitis [49].
Conclusion
Acute pancreatitis in pregnancy is a rare but life-threatening event. A high index of suspicion is required for making prompt diagnosis at presentation and putting a timely adequate management plan in action of the pregnant woman with pancreatitis will improve the maternal-fetal outcome. Mild to moderate pancreatitis in pregnancy can resolve effectively with fluid therapy, nutrition, analgesia and judicious use of antibiotics. However, if diagnosis is missed or delayed, it can lead to unfortunate complications for the mother and mostly for the fetus. The rate of pre-term delivery in PAAP is about 20% whereas maternal mortality is now less than 1%. Fetal loss is associated with severity of the disease. It is now accepted dogma that pain control, aggressive fluid resuscitation with isotonic crystalloid solution and early nutrition are the main mainstay of management of PAAP irrespective of the underlying pathophysiology or the severity of the disease.
To minimize maternal and fetal mortality and morbidity diligent decision-making related to the termination of pregnancy and the management of acute pancreatitis with its underlying pathology will require a multidisciplinary team approach. This should include obstetrician, surgeon, interventional gastroenterologist, radiologist and ICU staff. Preventative measures through nurse education in pre and perinatal period should be encouraged, especially monitoring patient’s weight, lowering their lipid profile, promoting a low-fat diet and emphasizing abstention from alcohol during pregnancy.
Acknowledgement
The authors are thankful to Qatar National Library for the support of open access to this article.
References
- Abdullah B, Pillai TK, Cheen LH, Ryan RJ (2015) Severe acute pancreatitis in pregnancy. Case Rep Obstet Gynecol 2015: 239068.
- Zhang DL, Huang Y, Yan LI, Phu A, Ran X, et al. (2013) Thirty-eight cases of acute pancreatitis in pregnancy: A 6-year single center retrospective analysis. J Huazhong Univ Sci Technolog Med Sci 33(3): 361-367.
- Igbinosa O, Poddar S (2013) Pitchumoni C Pregnancy associated pancreatitis revisited. Clin Res Hepatol Gastroenterol 37(2): 177-181.
- Luo L, Zen H, Xu H, Zhu Y, Liu pI, et al. (2018) Clinical characteristics of acute pancreatitis in pregnancy: experience based on 121 cases. Arch Gynecol Obstet 297(2): 333-339.
- Sun L, Li W, Geng Y, Shen B, Li J (2011) Acute pancreatitis in pregnancy. Acta Obstet Gynecol Scand 90(6): 671-676.
- Ducarme G, Maire F, Chatel P, Luton D, Hammel P (2014) Acute pancreatitis during pregnancy: A review. J Perinatol 34(2): 87-94.
- Terzhumanov R, Uchikov A, Uchikova E, Milchev H, Dimov R, et al. (2004) [Acute pancreatitis and pregnancy--analysis of a 10 year period of time]. Akush Ginekol (Sofiia) 43(7): 9-12.
- Zhu Y, Pan X, Zeng H, He W, Xia L, et al. (2017) A study on the etiology, severity, and mortality of 3260 patients with acute pancreatitis according to the revised atlanta classification in jiangxi, China over an 8-year period. Pancreas 46(4): 504-509.
- Zhang R, Deng L, Jin T, Zhu P, Shi N, et al. (2019) Hypertriglyceridaemia-associated acute pancreatitis: diagnosis and impact on severity. HPB (Oxford) 21(9): 1240-1249.
- Maringhini A, Ciambra M, Baccelliere P, Raimondo M, Orlando A, et al. (1993) Biliary sludge and gallstones in pregnancy: incidence, risk factors, and natural history. Ann Intern Med 119(2): 116-120.
- Kern F, Everson GT, DeMark B, McKinley C, Showalter R. et al. (1981) Biliary lipids, bile acids, and gallbladder function in the human female. Effects of pregnancy and the ovulatory cycle. J Clin Invest 68(5): 1229-1242.
- Kondrackiene J, Kupcinskas L (2008) Liver diseases unique to pregnancy. Medicina (Kaunas) 44(5): 337-345.
- Whitcomb DC, Yadav D, Adam S, Hawes RH, Brand RE, et al. (2008) Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American pancreatitis study 2 (NAPS2). Pancreatology 8(4-5): 520-531.
- Herrera MP, Oaks JB, Brensilver J (2018) A case of acute pancreatitis in hemolysis, elevated liver enzymes, and low platelets syndrome. Cureus 10(6): e2877.
- Zarnescu NO, Barbu ST, Zarnescu VEC, Costea R, Neagu S. (2015) Management of acute pancreatitis in the early stage. Maedica (Bucur) 10(3): 257-263.
- Larusch J, Whitcomb DC (2011) Genetics of pancreatitis. Curr Opin Gastroenterol 27(5): 467-474.
- Schorn S, Güralp O. Tieftrunk E, Friess H, Demir IE (2015) Pain management in acute pancreatitis. Pancreapedia: Exocrine Pancreas Knowledge Base, pp.1-13.
- Huang JL, Chen WK, Lin CL, Kao CH, Shih HM (2020) Preeclampsia and the risk of pancreatitis: a nationwide, population-based cohort study. Gastroenterol Res Pract 2020: 3261542.
- Lerch MM, Weidenbach H, Gress TM, Adler G (1995) Effect of kinin inhibition in experimental acute pancreatitis. Am J Physiol 269(4 Pt 1): G490-499.
- Sendler M, Dummer A, Weiss FU, Krüger B, Wartmann T, et al. (2013) Tumour necrosis factor alpha secretion induces protease activation and acinar cell necrosis in acute experimental pancreatitis in mice. Gut 62(3): 430-439.
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, et al. (2013) Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 62(1): 102-111.
- Wang GJ, Gao CF, Wei D, Wang C, Ding SQ (2009) Acute pancreatitis: etiology and common pathogenesis. World J Gastroenterol 15(12): 1427-1430.
- Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, et al. (2005) Pathophysiology of acute pancreatitis. Pancreatology 5(2-3): 132-144.
- Foster BR, Jensen KK, Bakis G, Shaaban AM, Coakley FV (2016) Revised Atlanta classification for acute pancreatitis: A pictorial essay. Radiographics 36(3): 675-687.
- Jasdanwala S, Babyatsky M (2015) A critical evaluation of serum lipase and amylase as diagnostic tests for acute pancreatitis. Integrative Molecular Medicine 2(3): 189-195
- Imamura T, Tanaka S, Yoshida H, Kitamura K, Ikegami A, et al. (2002) Significance of measurement of high-sensitivity C-reactive protein in acute pancreatitis. J Gastroenterol 37(11): 935-938.
- Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL (2016) Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 316(9): 952-961.
- Barthel JS, Chowdhury T, Miedema BW (1998) Endoscopic sphincterotomy for the treatment of gallstone pancreatitis during pregnancy. Surg Endosc 12(5): 394-399.
- Nesbitt TH, Kay HH, Mccoy MC, Herbert WN (1996) Endoscopic management of biliary disease during pregnancy. Obstet Gynecol 87(5 Pt 2): 806-809.
- Jamidar PA, Beck GJ, Hoffman BJ, Lehman GA, Hawes RH, et al. (1995) Endoscopic retrograde cholangiopancreatography in pregnancy. Am J Gastroenterol 90(8): 1263-1267.
- Woodhead N, Caddick V, Morad S, Mylvaganam S, Nkwam O (2019) Surgical causes of acute abdominal pain in pregnancy. Obstet Gynaecol 21(1): 27-33.
- Zachariah SK, Fenn M, Jacob K, Arthungal SA, Zachariah SA (2019) Management of acute abdomen in pregnancy: current perspectives. Int J Womens Health 11: 119-34.
- Goldberg AS, Hegele RA (2012) Severe hypertriglyceridemia in pregnancy. J Clin Endocrinol Metab 97(8): 2589-2596.
- Lindkvist B, Appelros S, Regner S, Manjer J (2012) A prospective cohort study on risk of acute pancreatitis related to serum triglycerides, cholesterol and fasting glucose. Pancreatology 12(4): 317-324.
- Sezgin O, Ozdogan O, Yaras S, Ucbilek E, Altintas E (2019) Evaluation of hypertriglyceridemia-induced acute pancreatitis: A single tertiary care unit experience from Turkey. Turk J Gastroenterol 30(3): 271-277.
- Venkatesh NR, Vijayakumar C, Balasubramaniyan G, Kandhasamy SK, Sundaramurthi S, et al. (2020) Comparison of different scoring systems in predicting the severity of acute pancreatitis: A prospective observational study. Cureus 12(2): e6943.
- Srinivasan G, Venkatakrishnan L, Sambandam S, Gursharan S, Kaur M, et al. (2016) Current concepts in the management of acute pancreatitis. J Family Med Prim Care 5(4): 752-758.
- Beyer GM, Julia M, Simon P, Lerch MM (2016) Fluid resuscitation in acute pancreatitis. Pancreapedia: Exocrine Pancreas Knowledge Base, pp. 1-6.
- Trikudanathan G, Vege SS (2014) Current concepts of the role of abdominal compartment syndrome in acute pancreatitis-an opportunity or merely an epiphenomenon. Pancreatology 14(4): 238-243.
- Jacobson BC, Vliet MBV, Hughes MD, Maurer R, McManus K, et al. (2007) A prospective, randomized trial of clear liquids versus low-fat solid diet as the initial meal in mild acute pancreatitis. Clin Gastroenterol Hepatol 5(8): 946-951.
- Bakker OJ, Brunschot SV, Santvoort HCV, Besselink MG, Bollen TL, et al. (2014) Early versus on-demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med 371(21): 1983-1993.
- Isenmann R, Runzi M, Kron M, Kahl S, Kraus D, et al. (2004) Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology 126(4): 997-1004.
- Maravi PE, Gener J, Alvarez LF, Olaechea P, Blanco A, et al. (2003) Early antibiotic treatment (prophylaxis) of septic complications in severe acute necrotizing pancreatitis: a prospective, randomized, multicenter study comparing two regimens with imipenem-cilastatin. Intensive Care Med 29(11): 1974-1980.
- Azab M, Bharadwaj S, Jayaraj M, Annie HS, Pejman S, et al. (2019) Safety of endoscopic retrograde cholangiopancreatography (ERCP) in pregnancy: A systematic review and meta-analysis. Saudi J Gastroenterol 25(6): 341-354.
- Tenner S, Baillie J, Dewitt J, Vege SS (2013) American college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 108(9): 1400-1416.
- Tang SJ, Mayo MJ, Frias ER, Armstrong L, Tang L et al. (2009) Safety and utility of ERCP during pregnancy. Gastrointest Endosc 69(3 Pt 1): 453-461.
- Gupta N, Ahmed S, Shaffer L, Cavens P, Blankstein J (2014) Severe hypertriglyceridemia induced pancreatitis in pregnancy. Case Rep Obstet Gynecol 2014: 485493.
- Sun Y, Fan C, Wang S (2013) Clinical analysis of 16 patients with acute pancreatitis in the third trimester of pregnancy. Int J Clin Exp Pathol 6(8): 1696-1701.
- Tang M, Xu JM, Song SS, Mei Q, Zhang LJ (2018) What may cause fetus loss from acute pancreatitis in pregnancy: Analysis of 54 cases. Medicine (Baltimore) 97(7): e9755.
© 2023 Elsayed YMH. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






