- Submissions

Full Text
Surgical Medicine Open Access Journal
Thyrotoxicosis in Silent Passed Myocardial Infarction with Responded Atrial Fibrillation in an Elderly Severely Anemic Patient- Interpretation and Outcome
Elsayed YMH*
Critical Care Unit, Egyptian Ministry of Health (MOH), Egypt
*Corresponding author:Yasser Mohammed Hassanain Elsayed Critical Care Unit, Kafr El-Bateekh Central Hospital, Damietta Health Affairs, Egyptian Ministry of Health (MOH), Damietta, Egypt
Submission: June 19, 2023Published: June 27, 2023

ISSN 2578-0379 Volume5 Issue3
Abstract
Rationale: Atrial fibrillation occurs in up to 15% of patients with hyperthyroidism compared to 4% of
people in the general population. Silent myocardial ischemia may occur in the absence of chest pain.
Silent myocardial ischemia would carry serious sequelae in delayed diagnosis and the appearance
of complications. Atrial fibrillation is the most commonly encountered clinical arrhythmia, often
complicating acute myocardial infarction. It contributes to high rates of in-hospital adverse events.
Anemia may be a risk factor for tachycardia.
Patient concerns: A 65-year-married male, worker, and Egyptian, patient was presented to the critical
care unit with atrial fibrillation, silent myocardial infarction, and thyrotoxicosis.
Diagnosis: A 65-year-married male, worker, and Egyptian, patient was presented to the critical care unit
with atrial fibrillation, silent myocardial infarction, and thyrotoxicosis.
Interventions: Electrocardiography, oxygenation, blood transfusion, and. abdominal ultrasound
Outcomes:Dramatic electrocardiographic and clinical stabilization was the result.
Keywords:Silent coronary heart disease; Infarction; Arrhythmia; Anemia; Thyrotoxicosis; Atrial fibrillation; Elderly
Abbreviations:AF: Atrial Fibrillation; AMI: Acute Myocardial Infarction; DCC; Cardioversion; ECG: Electrocardiogram; HR; Heart Rate; ICU: Intensive Care Unite; MI: Myocardial Infarction; NSR: Normal Sinus Rhythm; O2: Oxygen; SGOT: Serum Glutamic-Oxaloacetic Transaminase; SGPT: Serum Glutamic- Pyruvic Transaminase; SMI; Silent Myocardial Ischemia; STEMI: ST-Segment Elevation Myocardial Infarction; VR: Ventricular Rate
Introduction
Atrial Fibrillation (AF) is a common complication of Acute Myocardial Infarction (AMI) [1,2]. It has a role in high rates of in-hospital adverse events. AF is the most commonly documented arrhythmia post-AMI with an incidence between 6 and 21% [3]. New-onset AF post-AMI is strongly correlating with in-hospital complications of AMI and higher short-term readmission rates [1]. The overall incidence of AF after AMI was 10.8%. Rates of new-onset AF increased from 1999 to 2003 (9.8% to 13.2%) but subsequently decreased. Development of AF during hospitalization for AMI was associated with higher rates of readmission within 30 days after discharge (21.7% vs. 16.0%) [1]. Past studies suggest the link of AF during early hospitalization for AMI to the risk of Sudden Cardiac Death (SCD) [2], especially more than 30 days after MI [2]. Acute myocardial infarction usually has no apparent symptoms [4]. Silent Myocardial Ischemia (SMI) can occur in the absence of chest pain or other anginal equivalent symptoms, e.g., dyspnea, nausea, and diaphoresis [4]. SMI was initially described at the beginning of the 20th century [5].
Atherosclerosis is a silent progressive process, which can usually progress with advancing age and is accelerated by smoking, dyslipidemia, hypertension, and DM. Asymptomatic atherosclerosis may lead to an acute event, mostly due to plaque rupture and secondary thrombosis, most often, an acute heart event and an ischemic stroke [6]. About 70% to 80% of acute transient ischemic events do not present with angina. Controlling modifiable risk factors and changing lifestyle can improve the quality of life [7]. Beta-blockers are the drug of choice. Aspirin and statins are also used [4]. AF occurs in up to 15% of patients with hyperthyroidism vs. 4% of the general population. It is more frequent In Triiodothyronine (T3) toxicosis [8], in men (2.86%) [9], and in the elderly [8] with hyperthyroidism. Several suggested mechanisms for the effect of thyroid hormones on AF risk include elevation of LA pressure secondary to increased LV mass and impaired LV relaxation, ischemia due to increased resting Heart Rate (HR) and increased atrial ectopic activity [8]. AF for more than one year and advanced age were likely to need intervention in the long run, probably reflecting the coexistence of IHD in these hyperthyroid patients with AF [9].
Case Presentation
A 65-year-married male, heavy smoker, Egyptian, worker patient was presented to the intensive care unit (ICU) with palpitations for 3 days. Dizziness and profuse sweating were the associated symptoms. There is a recent history of socio-familial troubles. Upon general physical examination; generally, the patient seems to be tetany, irritable, and distressed. GCS was 15. An irregular rapid heart rate of 125 bpm, blood pressure of 140/70mmHg, respiratory rate of 18bpm, a temperature of 36 °C, and pulse oximeter of O2 saturation of 95% were reported. There is noted asymmetrical nontender bilateral thyroid enlargement. No more relevant clinical data were noted during the clinical examination. Urgent ECG tracing was done on the presentation in the ICU showing AF (VR; 128) with ST-segment elevation, pathological Q-wave in the inferior leads (II, III, and aVF), and reciprocal ST-segment depression in the anterior leads (I, aVL, and V2-5 (Figure 1A). Right ECG tracing (V3R and V4R) was done within 3 minutes of the above tracing and showed AF (VR; 122) with ST-segment elevation and pathological Q-wave in V3R and V4R leads (Figure 1B).
Figure 1:A- ECG tracing was done on the ICU admission and showed AF (VR; 128) with ST-segment elevation (red arrows)
and pathological Q-wave (light blue arrows) in the inferior leads (II, III, and aVF) and reciprocal ST-segment depression
in the anterior leads (I, aVL, and V2-5; lime arrows).
B- Right ECG tracing (V3R and V4R) was done within 3 minutes of the above tracing and showed AF(VR;122)
with ST-segment elevation (red arrows) and pathological Q-wave (light blue arrows) in V3R and V4R leads.
C- ECG tracing was done within 2.5 hours after treatment and showed AF (VR; 125) with complete resolution
of the above ST-segment elevation (red arrows) and disappearance of reciprocal ST-segment depression. There is
evidence of tremor artifacts (small golden arrows).
D- ECG tracing was done within 2.5 hours after treatment and showed NSR (green arrows; VR; 74) with T-wave
inversion in II, III, AVF, and V6 leads (pink arrows).
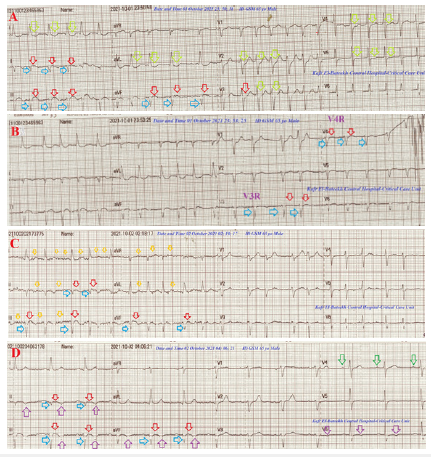
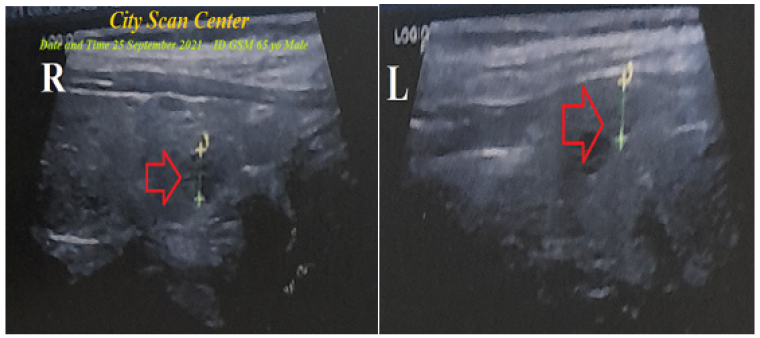
Figure 2:Thyroid ultrasound was done within 5 days before the ICU admission and showed right and left thyroid nodules that are surrounded by a radiolucent rim (Halo sign; red arrow).
The patient was admitted and treated at ICU with O2 inhalation (100%, by nasal cannula, 5L/min) by systemic oxygen line, 4 oral aspirin tablets (75mg, then once daily), 4 oral clopidogrel tablets (75mg, then once daily), oral propranolol tablet (10mg; twice daily), amiodarone HCL tablets (200 mg, twice daily), nitroglycerin retard capsule (2.5mg twice daily), warfarin tablets (5mg, once daily), atorvastatin (20mg once daily), and enoxaparin SC 60mg (twice daily). ECG tracing was repeated within 2.5 hours after treatment and showed still AF (VR; 125) with complete resolution of the above ST-segment elevation and disappearance of reciprocal STsegment depression. There is evidence of tremor artifacts (Figure 1C). The initial Complete Blood Count (CBC) showed; Hb was 7.4g/ dl, RBCs; 4.48*103/mm3, WBCs; 5.5*103/mm3 (Neutrophils; 65.3 %, Lymphocytes: 27.9%, Monocytes; 6.8%, Eosinophils; 0% and Basophils 0%), Platelets; 246*103/mm3. SGPT was normal (41U/L). SGOT was normal (25U/L). Serum creatinine was slightly high (1.56mg/dl) and blood urea was normal (37mg/dl). The troponin I was high (15.57ng/ml). Free T3 was high (5.3pg/dl). Free T4 was high (2.8ng/dl). TSH was low (0.1mU/l). Three fresh units of blood transfusion were given. ECG tracing was done within 2.5 hours after treatment and showed NSR (VR; 74)with T-wave inversion in II, III, AVF, and V6 leads (Figure 1D).
The echocardiography report showed inferior and right ventricular hypokinesia with an ejection fraction of (EF; 51%). A thyroid ultrasound was done within 5 days before the ICU admission and showed right and left thyroid nodules that are surrounded by a radiolucent rim (Figure 2). The patient on discharge was continued on captopril tablet (12.5mg once daily), aspirin tablet (75mg, once daily), clopidogrel tablet (75mg, once daily), nitroglycerin retard capsule (2.5mg twice daily), oral propranolol tablet (10mg; twice daily), amiodarone HCL tablets (200mg, once daily), and atorvastatin (20mg once daily) until discharged on the 5th day. Passed silent myocardial infarction with atrial fibrillation, severe anemia, and thyrotoxicosis in the elderly patient was the most probable current diagnosis. The patient was advised to have future outpatient cardiovascular, hematology, and endocrinology followups.
Discussion
A. Overview: An elderly married male, heavy smoker,
Egyptian, worker patient was presented to the ICU with
passed silent myocardial infarction, AF, severe anemia, and
thyrotoxicosis.
B. The primary objective: for my case study was the presence
of a patient with passed silent myocardial infarction, AF, severe
anemia, and thyrotoxicosis in the ICU.
C. The secondary objective: for my case study was the
question of; how did you manage the case at ICU?
Associated documented pathological Q-waves with still mild ST-segment elevation in both inferior leads (II, III, and aVF) (Figure 1A) and right ventricle (RV) leads (V3R and V4R) (Figure 1B) with elevated troponin indicate the presence of acute inferior and RV infarction. Silent myocardial infarction can be detected by ECG ST-segment changes on ECG, Reversible Regional Wall Motion Abnormalities (RWMA), or perfusion defects on scintigraphy imaging [4]. Elderly age in the current case may be a cause of delayed early hospital presentation for the patient. This is preventing the chance of being given a suitable thrombolytic therapy for AMI. So, an absence of chest pain in SMI increases morbidity and mortality since patients do not seek medical service in a timely fashion [6].
Diabetes Mellitus (DM), perioperative elderly, women, obstructive sleep apnea, and critically ill patients in the ICU admitted for non-cardiac causes is a significant risk factors for SMI [1]. The current echocardiography evidence of inferior and right ventricular hypokinesia with diminished an ejection fraction of (EF; 51%) may have a causal role in the present AF. Thyrotoxicosis is also an established factor. The prognostic value of AF with MI is still controversial [2]. Predictors of AF in the setting of SMI include advanced age, Heart Failure (HF) symptoms, and depressed LV function. AF in patients hospitalized for AMI has serious adverse prognostic implications regarding in-hospital, but also long-term mortality [3].
The dramatic reversal of clinical and electrocardiographic happened after both traditional anti-ischemic measures, rate, and rhythm control for AF. The initial treatment is a pharmacologic control of HR and to start antithyroid therapy as quickly as possible. Attempted Cardioversion (DCC) should be deferred until approximately the fourth month of maintaining a euthyroid state because more than 56% of AF spontaneously reverts to NSR when the thyroid hormone levels start to decline. Elective DCC for persistent AF is highly effective [9]. Treatment of hyperthyroidism yields conversion to sinus rhythm in up to two-thirds of patients [8].
Digitalis toxicity was the possible differential diagnosis for the current case study. But the current history had no digitalis prescription. I can’t compare the current case with similar conditions. There are no similar or known cases with the same management for near comparison. There are no limitations of the current study.
Conclusion and Recommendation
The mind orientation for the physician in atypical presentation of silent Myocardial Infarction in some subgroups such as the elderly, diabetes mellitus, women, obstructive sleep apnea, and critically ill patients is critical and pivotal. The elderly is a significant risk factor for silent myocardial infarction. Silent myocardial infarction, subsequent left Ventricular Dysfunction, Thyrotoxicosis, and elderly constellation risk factors Atrial Fibrillation.
Acknowledgment
I wish to thank my wife to save time and improving the conditions for supporting me. I want to thank the nurse team of the intensive care unit in Kafr El-Bateeck Central Hospital to give me extra copies of the ECG to help me.
References
- Kundu A, O'Day K, Shaikh AY, Lessard DM, Saczynski JS, et al. (2016) Relation of atrial fibrillation in acute myocardial infarction to in-hospital complications and early hospital readmission. Am J Cardiol 117(8): 1213-128.
- Jabre P, Jouven X, Adnet F, Thabut G, Suzette J, et al. (2011) Atrial Fibrillation and death after myocardial infarction a community study. Circulation 123(19): 2094-2100.
- Schmitt J, Duray G, Bernard JG, Hohnloser SH (2009) Atrial fibrillation in acute myocardial infarction: A systematic review of the incidence, clinical features and prognostic implications, European Heart Journal 30(9): 1038-1045.
- Gul Z, Makaryus AN (2023) Silent Myocardial Ischemia. Stat Pearls, Bookshelf ID: NBK536915.
- Cohn PF (1987) Silent ischemia: A timely aspect in coronary artery disease. Herz 12(5): 314-377.
- Scheen AJ (2018) From atherosclerosis to atherothrombosis: from a silent chronic pathology to an acute critical event. Rev Med Liege 73(5-6): 224-228.
- Aronow WS (2003) Silent MI. Prevalence and prognosis in older patients diagnosed by routine electrocardiograms. Geriatrics 58(1): 24-26, 36-38, 40.
- Dabrowa AB, Mikhailidis DP, Rysz J, Banach M (2009) The mechanisms of atrial fibrillation in hyperthyroidism. Thyroid Research 2(4): 1-7.
- Shimizu T, Koide S, Noh JY, Sugino K, Ito K, et al. (2002) Hyperthyroidism and the management of atrial fibrillation. Thyroid 12(6): 489-493.
© 2023 Elsayed YMH. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






