- Submissions

Full Text
Surgical Medicine Open Access Journal
Assessment of Spontaneous Pneumomediastinum: Experience with 15 Patients
Mehmet Sarikaya* and Fatma Ozcan
Department of Pediatric Surgery, Selcuk University Medical Faculty, Alaeddin Keykubat Campus, Turkey
*Corresponding author:Mehmet Sarikaya, Department of Pediatric Surgery, Selcuk University Medical Faculty, Alaeddin Keykubat Campus, Turkey
Submission: May 15, 2022Published: May 23, 2022

ISSN 2578-0379 Volume5 Issue2
Introduction
Pneumomediastinum, also known as “mediastinal emphysema”, is the presence of air in the mediastinum. Spontaneous Pneumomediastinum (SPM) is the presence of free air in the mediastinum without any causal factor such as trauma, mechanical ventilation, or iatrogenic injury [1]. SPM is a rare entity that is often triggered by activities that cause acute changes in intrathoracic pressure, such as childbirth, strenuous exercise, vomiting, and coughing. It was first described in adults in 1939 and is much less common in children [2]. Free air may leak from the ruptured alveoli into the pulmonary interstitium and then reach the mediastinum through the bronchovascular sheaths. It can migrate from the mediastinum to the neck, esophagus, submandibular space, and retropharyngeal space. Its incidence in the pediatric population ranges from 1/8000 to 1/15000 [3]. There are two peak points for the disease, 6 months to 4 years old and 15 to 18 years old, and it is more common in male patients [4,5]. While tallness and thinness are known risk factors for spontaneous pneumothorax, no significant demographic features have been identified for SPM. There is no consensus or standardization for the treatment of the disease and the management of the disease process. In this study, we aimed to share our 10-year experience in the management of SPM cases in children.
Methods
The files of patients who were treated in our clinic for spontaneous pnomomediastinum between January 2013 and January 2023 were retrospectively reviewed. Fifteen pediatric patients whose diagnosis was confirmed by thorax tomography were included in the study. In our study, traumatic cases, cases that developed SPM during or during intubation, cases that underwent cervical or thoracic surgery, and cases with tracheostomy were excluded. In addition to the demographic data of the patients; The etiology of SPM, treatment process, admission complaints, clinical features, length of hospital stay, transition to oral feeding, imaging studies were examined in detail.
Results
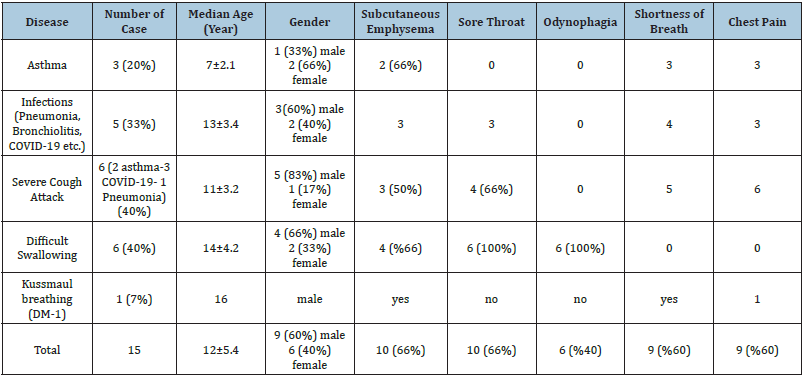
The mean age of the 15 SPM patients included in the study was 12±5.4, and nine (60%) were male and six (40%) were female. Demographic and clinical characteristics of the patients are given in (Table 1). Chest x-ray and then computed thorax tomography were performed in all cases (Figure 1). Etiology was asthma in three (20%) patients; pneumonia in five (33%) patients; Difficult swallowing was present in six (40%) patients. One (7%) patient developed SPM due to kuss-maul respiration resulting from diabetic keatoacidosis. Three of the five cases with pneumonia had COVID-19 pneumonia. Subcutaneous emphysema in 10 (66%) cases; six (40%) had difficulty swallowing; 10 (66%) had sore throat; Nine (60%) of them were found to have shortness of breath. While all of the cases were followed in at least 2nd level intensive care unit for an average of 3±1.2 days, the average hospital stay of the cases was 5±1.3 days. Esophagography was performed to exclude esophageal peroration in six. cases with swallowing difficulties. Contrast material leakage was not observed in the cases who underwent esophagography, but due to possible microperforation, they were followed up for 36±6 hours orally closed. We did not have a patient who had a chest tube or required surgery due to SPM. None of our patients died due to SPM.
Figure 1:
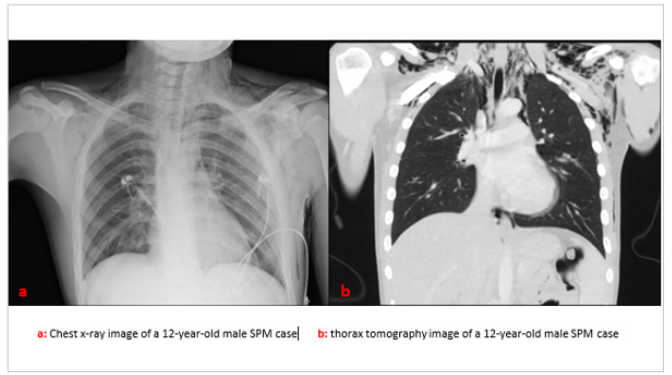
Table 1.Demographic and clinical characteristics of patients.
Discussion
Although SPM is very rare in children, it should be kept in mind in case of sudden onset chest pain and/or neck pain. The most common symptom described in the literature is chest pain [6]. Unlike the literature, the most common symptom in our series was sore throat in 66%, followed by chest pain in 60% and shortness of breath at the same rate. SPM has been associated with many conditions and triggers, such as asthma exacerbation, pneumonia, severe coughing or vomiting, and other activities associated with the Valsalva maneuver [7]. In our study, severe coughing attacks in six (40%) cases and difficulty swallowing in the same number of cases were prominent in the etiology. As in our series, no other study involving a high rate of difficult swallowing cases in etiology was found in the literature. In the esophagography taken for esophageal perforation, no finding suggestive of perforation was found in six cases with difficult swallowing in the etiology, but these cases were followed up for 36±6 hours orally closed due to the possibility of possible micro-perforation. Esophagoscopy was not performed on these patients because their esophagography was normal and their clinic was stable. We recommend that similar cases be followed up for at least 24 hours orally stop due to possible micro-perforation and associated mediastinitis.
After the Covid-19 pandemic, which started in Wuhan, China in December 2019 and affected the whole world, a large number of Covid-19-related SPM cases have been brought to the literature and this has been associated with a high risk of mortality [8,9]. In our series, Covid-19 pneumonia was present in three (20%) patients, but mortality did not develop in any of our cases. Agents such as H1N1, avian influenza A (H5N6), and human bocavirus have been implicated in the etiology of SPM [10-12]. One of the important etiological factors reported in the literature is asthma [13]. In our study, SPM developed during an asthma attack in three (20%) patients. Kussmaul breathing developed in Diabetic Ketoacidosis (DKA) cases have also been blamed for the etiology of SPM. In a study in which 747 DKA cases were examined, SPM was detected in the thorax tomography of 10 of these cases [14]. In our study, SPM developed due to Kussmaul respiration in a patient with DKA. Although chest x-ray is said to be sufficient for the diagnosis of the disease in most of the published SPM series, it may be insufficient for the diagnosis of mild cases [15]. In our series, chest tomography was performed in all cases in order to confirm the diagnosis, determine the severity of the disease, and evaluate additional pathologies.
The disease is usually self-limiting with a conservative approach, if there is priority in treatment, treatment of the underlying disease, oxygen support, painkillers and anti-inflammatory drugs are used [2]. In a review published by Gasser CR et al. [2] they stated that 25% of SPM cases were followed in the intensive care unit and the average hospital stay of the patients was four days [2]. In our study, all of the cases were followed up in the intensive care unit for an average of 3±1.2 days due to possible complications and were hospitalized for an average of 5±1.3 days. The most comprehensive systematic review of the disease was published by Alemu BN et al. [16] and the mortality rate was reported as 5.6% in this review [16]. The authors argue that mortal cases develop due to underlying diseases. No mortality was found in our series.
Conclusion
Cases with chest pain, sore throat, dysphagia, and dyspnea should be carefully examined for SPM. Although SPM is often selflimiting, it should be kept in mind that it can be mortal and patients should be followed up in intensive care conditions for the first 24-48 hours. Patients with sore throats and difficulty swallowing should be followed carefully for possible esophageal perforations and should not be rushed to switch to oral feeding.
References
- Potz BA, Chao LH, Ng TT, Okereke IC (2017) Clinical significance of spontaneous pneumomediastinum. Ann Thorac Surg 104(2): 431-435.
- Gasser CR, Pellaton R, Rochat CP (2017) Pediatric spontaneous pneumomediastinum: Narrative literature review. Pediatr Emerg Care 33(5): 370-374.
- Lee CY, Wu CC, Lin CY (2009) Etiologies of spontaneous pneumomediastinum in children of different ages. Pediatr Neonatol 50(5): 190-195.
- Chassagnon G, Favelle O, Derogis V, Cottier PJ (2015) Spontaneous pneumomediastinum due to the Macklin effect: Less is more. Intern Emerg Med 10(6): 759-761.
- Oudin C, Halbert C, Bosdure E, Arlaud K, Chabrol B, et al. (2008) Spontaneous pneumomediastinum in a 4-month-old boy. Arch Pediatr 15(11): 1652-1655.
- Asma M, Nesrine F, Ahmed BS, Sameh J, Saoussen CM, et al. (2019) Spontaneous pneumomediastinum: Experience in 13 patients. Respir Med Case Rep 28: 100946.
- Caceres M, Ali SZ, Braud R, Weiman D, Garrett HE (2008) Spontaneous pneumomediastinum: A comparative study and review of the literature. Ann Thoracic Surg 86(3): 962-966.
- Chowdhary A, Nirwan L, Ghanem AS, Arif U, Lahori S, et al. (2021) Spontaneous pneumomediastinum in patients diagnosed with COVID-19: A case series with review of literature. Acad Radiol 28(11): 1586-1598.
- Ganessane E, Devendiran A, Ramesh S, Uthayakumar A, Chandrasekar V, et al. (2023) Pneumomediastinum in COVID-19 disease: Clinical review with emphasis on emergency management. J Am Coll Emerg Physicians Open 4(2): e12935.
- Pekcan S, Gokturk B, Kucukapan HU, Arslan U, Fındık D (2014) Spontaneous pneumomediastinum as a complication in human bocavirus infection. Pediatr Int 56(5): 793-795.
- Zhang X, Wang J, Zeng Q, Wu X, Jiang S, et al. (2019) Spontaneous pneumomediastinum and subcutaneous emphysema in avian influenza A (H5N6) human pneumonia. Clin Case Rep 7(12): 2594-2595.
- Chekkoth SM, Supreeth RN, Valsala N, Kumar P, Raja RS (2019) Spontaneous pneumomediastinum in H1N1 infection: Uncommon complication of a common infection. J R Coll Physicians Edinb 49(4): 298-300.
- Girbés MT, Prat MM, Laguna DA, Mas S (2016) Spontaneous pneumomediastinum and subcutaneous emphysema as a complication of asthma in children: Case report and literature review. Ther Adv Respir Dis 10(5): 402-409.
- Xu WL, Sun LC, Zang XX, Wang H, Li WJ (2022) Spontaneous pneumomediastinum in diabetic ketoacidosis: A case series of 10 patients. World J Emerg Med 13(2): 141-143.
- Esayag Y, Furer V, Izbicki G (2008) Spontaneous pneumomediastinum: Is a chest X-ray enough? A single-center case series. Isr Med Assoc J 10(8-9): 575-578.
- Alemu BN, Yeheyis ET, Tiruneh AG (2021) Spontaneous primary pneumomediastinum: Is it always benign? J Med Case Rep 15(1): 157.
© 2023 Mehmet Sarikaya. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






