- Submissions

Full Text
Surgical Medicine Open Access Journal
Mid-Term Outcomes of the Perceval Sutureless Bioprosthesis for Aortic Infective Endocarditis
Victor X Mosquera1,4*, Adrian Muinelo-Paul1, Bárbara González-Oujo1, Oscar Pato-López3, Alberto Bouzas-Mosquera2,4 and José J Cuenca-Castillo1,4
1Department of Cardiac Surgery, Spain
2Department of Cardiology, Spain
3Department of Anesthesiology, Spain
4Instituto de Investigación Biomédica de A Coruña (INIBIC)
*Corresponding author:Victor X Mosquera, Department of Cardiac Surgery, Instituto de Investigación Biomédica de A Coruña (INIBIC) Complejo Hospitalario Universitario de A Coruña, A Coruña, Spain
Submission: January 11, 2023Published: February 09, 2023

ISSN 2578-0379 Volume5 Issue2
Abstract
Objective: The implantation technique of sutureless aortic bioprostheses shortens aortic crossclamp
and cardiopulmonary by-pass times, minimizes aortic annulus manipulation, and provides good
hemodynamics. These features might benefit the most patients with infective endocarditis.
Objectives: This study aims to analyze the mid-term clinical and hemodynamic outcomes of treating
complicated aortic infective endocarditis with Perceval (Livanova) bioprostheses.
Methods: Since January 2009, over 1300 consecutive patients underwent an aortic valve replacement
with a Perceval bioprosthesis. Among them, between July 2014 and June 2022, 36 patients were operated
on because of an aortic infective endocarditis.
Results: Median euroSCORE I was 34% (rank, 10.5%-85%) and median euroSCORE II was 15.8% (rank,
5.5%-79.2%). Preoperative echocardiographic evaluation revealed perivalvular extension in almost 60%
of the cases. All surgeries were performed on an urgent basis. The most frequently implanted prosthetic
size was L (41.7%), followed by size XL (27.8%), size M (25%) and size S (5.5%). An associated procedure
was necessary in 50% of the patients. Mean CPB and AXC times were 72.6±36.4 and 48.8±26.5 minutes,
respectively. Thirty-day mortality was 13.9%. Cumulative follow-up was 852.1 patient-months. Long-term
survival at 1 year and 5 years was 72.1% and 61.4%, respectively. There were no cases of endocarditis
relapse or new IE during the follow-up.
Conclusions: The Perceval sutureless aortic bioprosthesis provides an excellent alternative for surgical
treatment of challenging and time consuming complicated both native and prosthetic aortic IE. This
sutureless valve seems to allow a rapid, reproducible, and technically feasible repair, shortening both
aortic cross-clamp and cardiopulmonary by-pass times.
Keywords:Infective endocarditis; Sutureless valve; Aortic valve replacement; Perceval
Abbreviations:AXC: Aortic Cross-Clamp; CPB: Cardiopulmonary Bypass; ICU: Intensive Care Unit; IE: Infective Endocarditis; NVE: Native Valve Endocarditis; PVE: Prosthetic Valve Endocarditis; SU-AVR: Sutureless Aortic Valve Replacement; TEE: Transesophageal Echocardiogram
Introduction
Aortic rapid deployment and sutureless bioprostheses have opened up a new avenue for treatment of complicated scenarios of aortic Infective Endocarditis (IE). These prostheses allow to technically simplify the intervention, as the aortic annulus manipulation is minimized, and to reduce both Cardiopulmonary Bypass (CPB) and Aortic Cross-Clamp (AXC) times. Nowadays, there is growing evidence to suggest that Sutureless Aortic Valve Replacement (SU-AVR) with the Perceval (Livanova) valve associates with shorter CPB and AXC times as well as with improved hemodynamics compared to conventional stented bioprostheses [1,2]. These features might be especially appealing when operating on patients undergoing complex procedures, as multivalvular surgery [3] or surgery for Infective Endocarditis (IE) [3]. Patients with IE present with increased inflammatory and vasoactive mediators and, usually, with multiple comorbidities [4]. In fact, prolonged AXC and CPB times have been associated with mortality and development of severe complications after valvular surgery for IE [5]. Therefore, these patients will probably benefit most from shortening CPB and AXC, as well as the CPB-related inflammatory response. This study aims to analyze the short and mid-term clinical and hemodynamic outcomes of treating complicated native or prosthetic aortic IE with Perceval bioprostheses.
Materials and Methods
Since January 2009, over 1300 consecutive patients underwent an isolated or combined SU-AVR with either the Perceval S or the Perceval Plus (Livanova). Among them, between July 2014 and June 2022, 36 patients were operated on because of an aortic Native Valve Endocarditis (NVE) or Prosthetic Valve Endocarditis (PVE). All those patients had previously been evaluated by the institutional endocarditis team.
Ethical statement
The study was approved by the local research ethics committee (Comité Ético de Investigación Clínica de Galicia study identification number 261/2022).
Operative technique
In most cases a standard full median sternotomy was performed as usual with antegrade and retrograde cold blood cardioplegia repeated every 20min. In 4 cases, a superior ministernotomy was carried out through an inverted L incision of the sternum from the manubrium to the level of the third intercostal space with antegrade cold blood cardioplegia repeated every 20min. The aortic valve was accessed via a high transverse aortotomy. Complete excision of the aortic valve was performed, followed by aggressive debridement with removal of all infected and necrotic tissue. Subsequent reconstructive surgery with pericardial patches was performed to correct the defects, when necessary, before bioprosthesis implantation. Type of reconstruction varied according to the size of the defect. For small defects, direct closure was sufficient, whereas moderate to large-sized defects required an autologous or bovine patch (Figure 1).
Figure 1:(A) Aortic native valve endocarditis complicated with severe aortic regurgitation and subannular abscess under the non/left coronary commissure. (B) Reconstruction of the defect with a bovine pericardial patch. (C) implantation of a size L Perceval Plus bioprosthesis.
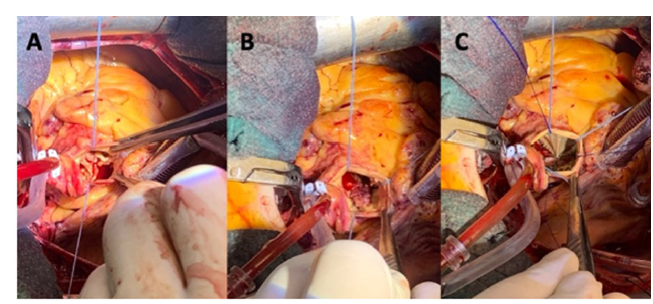
Sizing was performed as previously published by our group to minimize the rate of postoperative atrioventricular block [6]. The correct valve size was selected by measuring the white part of the obturator (valve sizer) that remained stable above the aortic annulus but could pass through the annulus itself while pushed only with a gentile force. The 3 guiding sutures were placed through the aortic annulus itself, at the nadir of each cusp and finally, the valve was ballooned at 4atm for 10s. In all patients, valve function was assessed through transesophageal echocardiography before weaning from cardiopulmonary bypass.
Statistical analysis
Descriptive results were presented as counts and percentages for categorical variables. Continuous data were described as mean and standard deviation or, in case of a skewed distribution, as median and range. Normal distribution of continuous data was evaluated by visual inspection of QQ plots and histograms as well as using the Kolmogorov–Smirnov test. Chi-squared with Yates’ correction or Fisher’s exact tests were used to compare proportions, whereas continuous variables were compared with the Student’s t-test. The Kaplan-Meier method was employed to analyze ‘time-todeath’ data. A p value of less than 0.05 was considered significant. Data analysis was performed with the SPSS version 25.0 and R Statistical Software version 3.2.0.
Results
The clinical and demographic characteristics of the 36 patients are shown in (Table 1) (69.4% males; mean age 69.4±10 years). The most common causative microorganisms were the Streptococcus spp. (33.3%), followed by the Staphylococcus spp. (30.6%) and the Enterococcus spp. (22.2%). The causative microorganisms are enlisted in (Table 2). Eighteen patients (50%) had a previous cardiac surgery. There were 2 patients with 3 previous cardiac procedures. There were not intravenous drug users in this series of IE. Among the 36 patients with IE, there were 11 patients (30.6%) suffering a PVE. More than 40% of the patients (n=15) were in preoperative NYHA class III-IV. In 6 cases, preoperative inotropic support was required, and 5 patients fulfilled the criteria for preoperative cardiogenic shock. Median euroSCORE I was 34% (range, 10.5%- 85%) and median euroSCORE II was 15.8% (range, 5.5%-79.2%).
Table 1.Demographics and clinical data (TIA: Transient Ischemic Attack)..
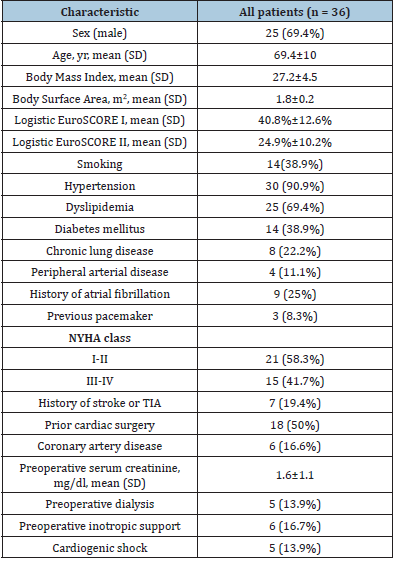
Table 2.Microbiological characteristics.
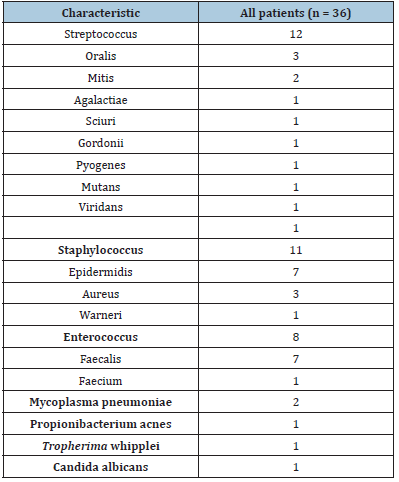
Table 3.Peri and intraoperative characteristics (MV: Mitral Valve).
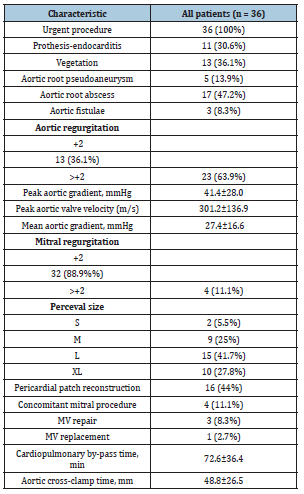
Preoperative echocardiographic evaluation revealed perivalvular extension of the IE in almost 60% of the cases (n=21) namely, an aortic abscess in 17 patients (47.2%); an aortic pseudoaneurysm in 5 patients (13.9%), and a fistula in 3 patients (8.3%). The perivalvular extension of the IE occurred most frequently in the noncoronary annulus. Vegetations on the aortic valve were also found in 13 patients (36.1%). Most patients had a double aortic valve dysfunction (n=19, 52.8%), while 14 patients (38.9%) suffered pure aortic regurgitation, and 3 patients aortic valve stenosis (8.3%). Echocardiographic data are displayed in (Table 3). All surgeries were performed on an urgent basis. The most frequently implanted valve size was L (41.7%), followed by size XL (27.8%), size M (25%) and size S (5.5%).
In 50% of the patients, an associated procedure was necessary. Sixteen patients (44.4%) required a pericardial patch to close an aortic root defect. Four patients (11.1%) underwent a concomitant mitral valve procedure namely, a mitral valve repair with an annuloplasty ring in 3 patients and a biological mitral valve replacement in the remaining patient. Mean CPB and AXC times were 72.6±36.4 and 48.8±26.5 minutes, respectively. Intraoperative variables are described in (Table 3). Six patients (16.7%) required surgical re-exploration because of either postoperative bleeding or cardiac tamponade. Three patients (8.3%) needed a permanent pacemaker due to atrioventricular block. However, these 3 patients had already developed preoperative atrioventricular block as a complication of the IE itself, requiring also preoperative admission to the Intensive Care Unit (ICU) because of these rhythm disturbances. There were 2 postoperative strokes. Thirtyday mortality was 13.9% (n=5). Three postoperative deaths were caused by a multisystem organ failure, and two cases due to low cardiac output syndrome. In the subgroup of patients who died, the median euroSCORE I and median euroSCORE II were 35.5% (range, 13.1%-84.8%) and 16.7% (range, 7.5%-40.4%), respectively.
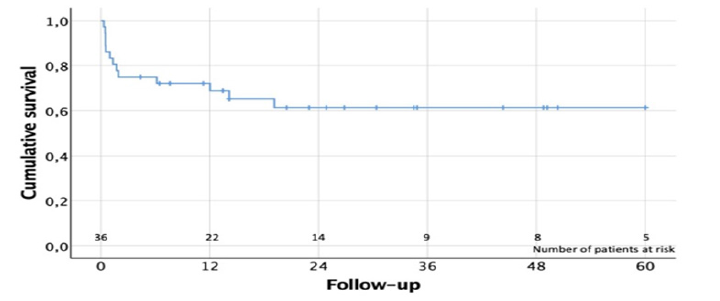
Although CPB and AXC times were longer in the subgroup of patients who died (n=5), there were no statistically significant association between mortality and CPB time (84±33.2min vs 70.8±37min, p= 0.46) and AXC time (48±28.2min vs 53.8±12.7min, p=0.656). There was no mortality among those patients undergoing a concomitant MV procedure. No statistically significant differences in mortality were identified between NVE and PVE (8.7% vs 23.1%, p=0.486). Univariate analysis suggested as potential risk factors of mortality: preoperative hemodynamic instability (50% vs 6.7%, p=0.031), and previous coronary bypass surgery (100% vs 8.8%, p=0.016). In the first month postoperative control TTE, mean and peak aortic gradients were 11±4.7 and 20.7±9.4mmHg, respectively. There were 3 cases with trivial-mild residual aortic regurgitation. First year postoperative control TTE showed a mean and peak aortic gradient of 10.8±3 and 21.4±9.3 mmHg, respectively. Mean and peak aortic gradients remained stable during the first postoperative year without significant changes (p=0.886, and p=0.960). At 1-year follow-up, only 2 cases of trivial-mild residual aortic regurgitation were identified. Echocardiographic data during the follow-up is shown in (Table 4). Cumulative follow-up was 852.1 patient-months. Long-term survival at 1 year and 5 years was 72.1% and 61.4%, respectively (Figure 2). There were no cases of endocarditis relapse or new IE during the follow-up.
Table 4.Follow-up echocardiographic data.
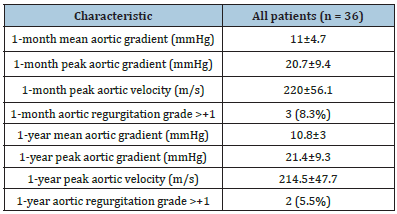
Figure 2:Kaplan-Meier survival curve.
Discussion
To the best of our knowledge, this is the largest and with longest follow-up single-center series describing the use of the Perceval sutureless bioprosthesis for the treatment of both aortic NVE and PVE. Our study demonstrates that SU-AVR with the Perceval valve is a feasible and especially attractive treatment for complicated aortic IE. On the one hand, the implantation of this sutureless valve permits a significant reduction of the CPB and AXC times, which poses an especially appealing advantage in high-risk patients with septic shock and/or hemodynamically unstable. On the other hand, in the daunting scenarios of periannular complications and multivalvular IE, these bioprostheses provide a simple treatment alternative, highly reproducible, technically achievable for most surgeons and with a remarkable procedural success rate. Finally, in our study, the Perceval prosthesis have demonstrated an outstanding hemodynamic performance with remarkably low gradients, no cases of LVOT obstruction, and no significant PVL (more than trivial-mild) not only at the 1-month but also at the 1-year postoperative controls.
Active IE is not only a heart valve disease but also a systemic condition and, therefore, in these patients we must weigh the potential detrimental impact of any associated coagulopathy, septic emboli, end-organ impairment, and a likely systemic inflammatory response syndrome. In fact, this critically ill subset of patients is depicted in our study by a mean logistic EuroSCORE I and EuroSCORE II of 40.8%±12.6% and 24.9%±10.2%, respectively. Moreover, half of the cases were reoperations and more than 50% of the patients required a concomitant procedure to the SU-AVR. Even in this appalling scenario, our observed 30-d mortality was 13.9% and the 1-year survival exceeded the 72%. Recently, Zubarevich et al. [7] have published a series 13 patients undergoing SU-AVR because of a complicated aortic IE. In that study, the authors reported a 30-d mortality of 23.1%, yet the expected mortality estimated by the logistic EuroSCORE I and EuroSCORE II was 47.9%±23.1% and 28.7%±22.0%, respectively. The presence of IE during cardiac valve surgery is associated with increased inflammatory response as evident by higher plasma cytokine levels and other inflammatory mediators, which is even related to a higher in-hospital mortality [4]. Additionally, it is also well-known that systemic inflammatory pathways are activated during CPB through activation of the complement system, coagulation pathways, leukocytes, and the release of inflammatory cytokines [8].
Therefore, in the treatment of IE, we as surgeons must seek not only to perform an extensive tissular debridement and posterior reconstruction of the affected heart valves, but also to avoid exacerbating the dysregulated inflammatory response and the impairment of other end-organ function by shortening the CPB time as much as possible. In fact, the current literature points out that prolonged CPB and AXC times are strongly associated with significant mortality and development of severe complications after valvular surgery for IE [5,9]. Salsano et al. [5] established the mortality cut-offs in >72min for AXC and >166min for CPB times. The rapid deployment technique of the Perceval allows to shorten both CPB and AXC times, even in the presence of significant impairment of the aortic annulus. In fact, in our study, 50% of the patients required an associated procedure to the SU-AVR, either a pericardial patch reconstruction of the aortic root or a mitral valve procedure. However, we registered a mean CPB and AXC times of 72.6±36.4 and 48.8±26.5 minutes, respectively.
Figure 3:Summary of the competitive advantages for the treatment of aortic infective endocarditis of the Perceval’s design and implantation technique (AXC: Aortic Cross-Clamp; CPB: Cardiopulmonary By-Pass; PVL: Paravalvular Leak).
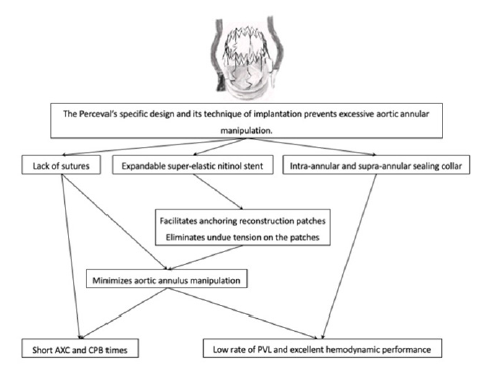
Similarly, in the previously cited recent study by Zubarevich et al. [7], the authors reported in their series of 13 patients a mean CPB and AXC times of 89.8±33.6 minutes and of 59.1±27.8 minutes, respectively. Not only many of the patients with IE are too sick to sustain long morbid operations, but also these patients would probably benefit the most from a hemodynamically good postoperative valvular performance and a low rate of PVL. Precisely, another of the advantages for the treatment of complicated IE of the Perceval sutureless valve’s technique of implantation is that it prevents excessive aortic annular manipulation. The lack of sutures and its expandable super-elastic stent facilitate the anchoring of reconstruction patches and eliminate undue tractions on them, thus reducing the rate of PVL. Likewise, the Perceval valve has been designed with both an intra-annular and supra-annular sealing collar. This configuration aids to minimize PVL in IE, especially when there is substantial annular destruction and tissue friability. In our study, more than the 60% of the patients have an aortic root complication, and yet there were no cases of significant PVL during the follow-up. (Figure 3) summarizes the advantages of the Perceval’s design and implantation technique for the treatment of aortic infective endocarditis.
About the theoretical shortcommings, several authors have pointed out that the “Achilles’ heel” of the Perceval sutureless valve is the higher rate of postoperative need for a permanent pacemaker [1,10,11]. Nonetheless, our group has already demonstrated that a modification of the Perceval’s implantation technique, especially regarding an adequate sizing, high placement of the guide sutures and non-aggressive ballooning, remarkably improves the postoperative atrioventricular block rate requiring implantation of permanent pacemaker [6]. The importance for lowering the postoperative pacemaker rate rests with an adequate sizing and implantation technique rather than with the bioprosthesis structural design. This fact has been also highlighted by other authors [12]. In current literature, patients with IE have been proven to present a higher need of permanent pacemaker, especially in those cases with perivalvular extension of the IE [13,14]. In our series, almost 60% of the patients suffered a perivalvular extension of the IE and developed severe preoperative rhythm disturbances that eventually led to the postoperative implantation of a pacemaker. Therefore, the 8.3% of postoperative pacemaker implantation can be better explained by the preoperative rhythm disturbances found as complications of the IE than by technique of implantation of the Perceval valve.
Limitations
This study presents the limitations inherent in any observational study. The primary limitation is that the number of patients enrolled in this study may be low and thus, this study may lack sufficient statistical power to detect certain clinically relevant effects. Additionally, IE is a continuous spectrum that includes from lowgrade aortic valve impairment to extensive aortic root destruction, as well as potential multivalvular involvement. This series does not reflect all degrees of aortic IE nor all clinical scenarios. A large comparative study between the Perceval and stented bioprostheses for the treatment of aortic IE is mandatory.
Conclusion
The Perceval sutureless aortic bioprosthesis provides an excellent alternative for surgical treatment of challenging and time-consuming complicated both native and prosthetic aortic IE. This sutureless valve seems to allow a rapid, reproducible, and technically feasible repair, shortening both AXC and CPB times. The implantation of this sutureless valve also avoids excessive aortic annular manipulation. Its characteristic double sheet design with supra and subannular sealing has proven to provide a low rate of postoperative PVL. The short surgical times and the outstanding hemodynamic outcomes might explain that our observed shortterm mortality was lower than the expected one, as well as our low observed rate of postoperative complications.
References
- Meco M, Montisci A, Miceli A, Panisi P, Donatelli F, et al. (2018) Sutureless perceval aortic valve versus conventional stented bioprostheses: Meta-analysis of postoperative and midterm results in isolated aortic valve replacement. J Am Heart Assoc 7(4): 006091.
- D'Onofrio A, Salizzoni S, Filippini C, Tessari C, Bagozzi L, et al. (2020) Surgical aortic valve replacement with new-generation bioprostheses: Sutureless versus rapid-deployment. J Thorac Cardiovasc Surg. 159(2): 432-442.
- Zubarevich A, Szczechowicz M, Zhigalov K, Osswald A, Van den Eynde J, et al. (2021) Sutureless aortic valve replacement in multivalve procedures. J Thorac Dis 13(6): 3392-3398.
- Diab M, Tasar R, Sponholz C, Lehmann T, Pletz MW, et al. (2020) Changes in inflammatory and vasoactive mediator profiles during valvular surgery with or without infective endocarditis: A case control pilot study. PLoS One 15(2): 0228286.
- Salsano A, Giacobbe DR, Sportelli E, Olivieri GM, Natali R, et al. (2018) Aortic cross-clamp time and cardiopulmonary bypass time: Prognostic implications in patients operated on for infective endocarditis. Interact Cardiovasc Thorac Surg 27(3): 328-335.
- González BM, Estévez CF, Pardo MP, Velasco GSC, Iglesias GC, et al. (2019) Surgical technique modifies the postoperative atrioventricular block rate in sutureless prostheses. J Thorac Dis 11(7): 2945-54.
- Zubarevich A, Rad AA, Szczechowicz M, Ruhparwar A, Weymann A (2022) Sutureless aortic valve replacement in high-risk patients with active infective endocarditis. Journal of Thoracic Disease 14(9): 3178-3186.
- Jufar AH, Lankadeva YR, May CN, Cochrane AD, Marino BR, et al. (2021) Renal and cerebral hypoxia and inflammation during cardiopulmonary bypass. Compr Physiol 12(1): 2799-2834.
- Chalmers J, Pullan M, Mediratta N, Poullis M (2014) A need for speed? Bypass time and outcomes after isolated aortic valve replacement surgery. Interact Cardiovasc Thorac Surg 19(1): 21-26.
- Vogt F, Pfeiffer S, Dell'Aquila AM, Fischlein T, Santarpino G (2016) Sutureless aortic valve replacement with Perceval bioprosthesis: Are there predicting factors for postoperative pacemaker implantation? Interact Cardiovasc Thorac Surg 22(3): 253-258.
- Woldendorp K, Doyle MP, Bannon PG, Misfeld M, Yan TD, et al. (2020) Aortic valve replacement using stented or sutureless/rapid deployment prosthesis via either full-sternotomy or a minimally invasive approach: A network meta-analysis. Ann Cardiothorac Surg 9(5): 347-363.
- Fabre O, Radutoiu M, Carjaliu I, Rebet O, Gautier L (2022) Recent improvement in operative techniques lead to lower pacemaker rate after Perceval implant. Interact Cardiovasc Thorac Surg 35(2): 182.
- Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, et al. (2015) 2015 ESC Guidelines for the management of infective endocarditis: The task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), European Association of Nuclear Medicine (EANM). Eur Heart J 36(44): 3075-3128.
- Habib G, Erba PA, Iung B, Donal E, Cosyns B, et al. (2019) Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: A prospective cohort study. Eur Heart J 40(39): 3222-3232.
© 2023 Victor X Mosquera. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






