- Submissions

Full Text
Surgical Medicine Open Access Journal
Skin Grafts in Plastic Surgery: Review Article
Jesús Nicolás LC1 and Nicolás Larco N2
1Medical Surgeon Pontifical Catholic University of Ecuador, Ecuador
2Faculty from Medicine University Central of the Ecuador, Ecuador
*Corresponding author: Jesús Nicolás Larco Coloma, Medical Surgeon Pontifical Catholic University of Ecuador, Ecuador
Submission: April 12, 2022Published: May 25, 2022

ISSN 2578-0379 Volume4 Issue5
Abstract
Skin grafts within plastic surgery are highly performed and constantly updated procedures, being the second most performed procedure worldwide in this specialty and, in turn, the one with the greatest comorbidities associated with poor management. The skin is the largest organ of the human body, which is why different plastic surgery techniques have been developed. This bibliographic review provides a historical, histological, morphofunctional and procedural overview of grafts. Which have great importance within the surgical unit.
Keywords: Skin grafts; Plastic surgery; Comorbidities; Human body; Surgical unit; Traumatic
Introduction
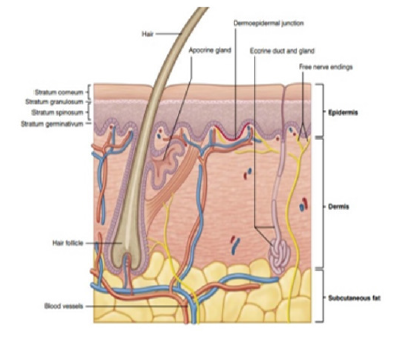
Skin grafts date back to 1500 BC in India, where they served as a covering for traumatic nasal amputations. At present, the use of skin grafts is extensive, they have become the most used procedure in reconstructive surgery for burns, oncological surgery and coverage in cases where the extension of the bloody area is very wide [1]. Over the years, new techniques have facilitated and improved the taking, processing, and placement of grafts, making skin grafting a fast, safe, and efficient procedure [1]. The skin corresponds to 8% of our body, with an average surface area of 1.7m², and it is also the heaviest organ that makes up the human body. The skin is an organ that changes its histological shape according to the area where it is located, so we have that it is thicker in areas such as the palms of the hands and soles of the feet and thinner in the eyelids and folds [1,2]. It is the main protection barrier against mechanical aggressions, provides thermoregulation and waterproofing, vitamin D production, absorption of ultraviolet radiation and allows the perception of tactile stimuli. The skin has 2 layers that are the epidermis and dermis: Epidermis: It is the most superficial and thin layer; it is covered by the stratum corneum. Keratinocytes, melanocytes, langerhans cells, and merkel cells are found here. It is subdivided into layers: basal or germinative, spinous or squamous, granular, lucid, and corneum [1] (Figure 1). Dermis: It is located below the epidermis, it is much thicker, it is made up of connective tissue crossed by numerous vessels and nerves, here the skin annexes are located. It is divided into two layers: Papillary dermis and reticular dermis [1,2]. Hypodermis: Corresponds to the subcutaneous cellular layer, it is the innermost layer of the skin composed of adipocytes [1,2].
Figure 1:Skin Anatomy taken from [7] surgery of the skin procedural dermatology.
Skin Graft Classification
According to the donor
Isografts are the transfer of tissue between two genetically identical individuals. Autograft is the transplant of skin from one area to another in the same individual. Allograft is the transplant that is performed between two subjects of the same species. Xenograft is the transplant that is performed between beings of different species [2,3].
Depending on the type of fabric
Skin graft, bone graft, nerve graft, tendon graft, vascular graft, cartilage graft, fat graft, fascia graft [2].
Skin graft
Defined as the transfer of dermal tissue from one place to another without its original irrigation, which requires several stages for its integration and viability. The grafts are classified into partial and total skin grafts, they are composed of epidermis and dermis, separating in its entirety from the donor area to the recipient area.
Split-thickness grafts, made up of epidermis and dermis, are
subdivided into:
Thins, ranging in thickness from 0.012 to 0.018 inches, include
the epidermis and papillary dermis. Medium, they are 0.018 to
0.022 inches thick and include the epidermis, papillary dermis, and
part of the reticular dermis. Thick, they range from 0.022 to 0.025
inches thick and encompass the epidermis, papillary dermis, and
a deeper portion of the reticular dermis [2]. The graft must meet
certain factors so that it heals properly in the recipient bed and
adheres to it, as follows:
i. Vascularization of the graft: It is important to take the
graft from an area of skin that has good vascularization.
ii. Vascularization of the recipient area: A recipient bed with
good quality tissue, free of bacterial biofilm and active infections,
is required.
iii. Graft thickness: The thinner the graft, the greater the
chance of revascularization and integration
iv. Graft metabolism: The size and thickness of the graft
influence the amount of energy required for its metabolism [1,2].
Skin graft apprehension
Grafting is a set of biological steps by which the graft is integrated into the recipient bed, this mainly depends on the speed with which the vascularity of the ischemic grafted tissue is restored [3].
This process happens in any type of graft:
Skin graft phases
i. Plasma inhibition: Lasts between 24 and 48 hours. It
begins with the formation of a fibrin layer that fixes the grafted
tissue in the recipient bed.
ii. Inoculation: Kissing-capillaries are produced, which is the
alignment of the capillaries of the graft with those of the recipient
bed.
iii. Revascularization: Anastomosis between the vessels of
the recipient bed with those of the graft. Neovascularization from
the recipient bed to the graft and combination of neo-vessels and
pre-existing vessels [3,4].
Partial skin grafts
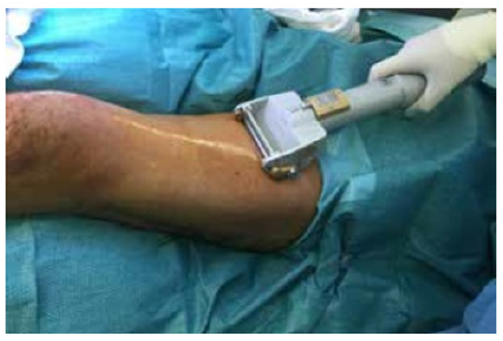
Partial skin grafts are harvested with a dermatome, either manual or electric, but can also be harvested with a scalpel or sterilized razor blades. The skin must be covered with lubricant, this allows the blade to slide easily into the tissue [4] (Figures 2 & 3). The donor area should be handled with a closed cure, Vaseline gauzes or active dressings such as hydrocolloids can be used to isolate the bloody area from bacteria, covering with sterile dressings and a cotton bandage, thus providing adequate temperature and humidity for healing [5]. The graft can be used lamellar or if necessary, it can be expanded, this process is carried out using a graft mesher. The expansion of the grafts allows to increase the coverage area using a smaller donor area, it can be expanded in a proportion of 1.3, 1.5, 3, 6, 9, the graft now gives a mesh appearance which is fixed in the bloody bed receiver [5]. The grafts can be fixed in several ways: titanium clips, suture threads or simply cover them with a sheet of Vaseline gauze, however, it will depend on the grafted area if it is mobile or difficult to access for the placement of dressings and bandages [6].
Figure 2:Partial skin graft harvest [6].
Receiving zone
Nerves, tendons and cartilage are not good receptor sites. The best receptor sites are the muscles, fat, fascia, dura and periosteum. The recipient areas must have granulation tissue in good condition, without necrotic tissue, foreign bodies or active bleeding, the raw surface is flat and with punctate bleeding. Bacterial balance: <105 microorganisms/gram of tissue [2,3].
Donor zone
Split-thickness grafts: Inner arm, buttocks, thighs, abdomen, back, anterior chest, scalp, leg (poor healing). Folds should be avoided because scarring causes contractures. Grafts should be taken that have a similar skin tone to that of the recipient bed.
Full-thickness grafts: Eyelids, postauricular, preauricular, supraclavicular, antecubital (elbow), wrist, hypothenar region, inguinal region, gluteal fold [6].
Total Skin Grafts
Total Skin Grafts
Figure 3:Donor area [4].
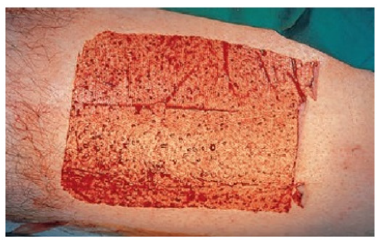
Figure 4:Graft incision [4].
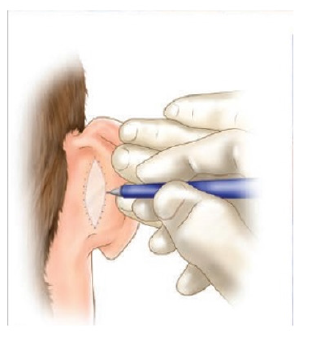
Figure 5:Graft fixation [4].
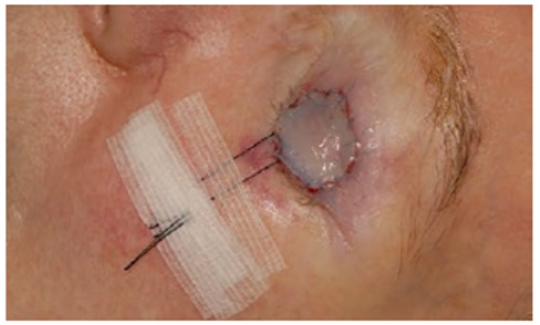
The recipient bed must be carefully prepared, control active bleeding. A template can be made over the defect and outlined with a felt pen to cut out the exact size and shape of the defect (Figure 4). The template is transferred to the donor site where it has been outlined with the marker pen. The donor area is then infiltrated with local anesthetic. The marked line is incised with a scalpel no. 15 and the extracted graft is lifted with atraumatic forceps and the scalpel blade [7,8]. The skin should be held under tension while the blade is used to stroke the skin, maintaining a constant depth that should be as shallow as possible. The defect may need to be converted to an ellipse to effect adequate wound closure [7]. The donor site is closed with a nylon suture, either as a simple running suture or simple stitches [8]. Subcutaneous cellular tissue is completely removed with blunt-tipped Westcott scissors, while holding the graft on the index finger of the non-dominant hand [9]. It is important to meticulously thin the graft and make small incisions with a scalpel so that possible hematomas can be drained, which are the main cause of loss due to lack of integration of the grafts [10]. Stitches are placed to fix the graft; the sutures are passed from the graft to the recipient skin edge (Figure 5). The graft is covered with Vaseline gauze. The sutures are joined together on the sponge to act as a cushion (Brown Dressing), this will help hemostasis and prevent the formation of hematomas in the recipient bed. The graft will be reviewed approximately 5 days after placement [11,12].
Conclusion
The management of skin grafts within plastic surgery is of the utmost importance, since it is a technique of daily use. The skin is the largest organ of the human body, so its structure and function lead to better handling of the grafts. The classification of grafts ranges from partial to complete, where it is important to know the different donor and recipient areas, as well as the different stages of the recipient area.
]References
- Bolognia JL, Schaffer JV, Cerroni L (2017) Dermatology. (4th edn), Elsevier Publishers, Netherlands, 2: 2752.
- Janis JE (2013) Essential of plastic surgery. Wound Grafting, (2nd edn), CRC Press, USA, pp. 1367.
- Jones JE, Nelson EA, Al-Hity A (2013) Skin grafting for venous leg ulcers. Cochrane Database of Systematic Reviews 2013(1).
- Leatherbarrow B (2020) Oculoplastic surgery. (3rd edn), The Journal of Laryngology & Otology, Thieme publishers, USA, 134(9): 846.
- Rubin JP, Neligan P (2017) Plastic surgery. (4th edn), Elsevier, Netherlands.
- Castela G (2016) Manual of ophthalmic plastic and reconstructive surgery. Portuguese Society of Ophthalmology, pp. 1-310.
- Robinson JK, Hanke CW, Siegel DM (2010) Surgery of the skin: Procedural dermatology. (2nd edn), Mosby Publishers, USA, pp. 1-868.
- Seher AF (2018) Wounds and healing.
- Sivakumar M, Mohapatra DP (2016) A review of flaps and their uses in reconstructive surgery. Journal of the Anatomical Society of India 69(2): 103-109.
- Zheng HP, Lin J, Xu YQ, Hu DQ (2019) Atlas of perforator flap and wound healing. Microsurgical reconstruction and cases. (1st edn), Springer Publishers, USA, pp. 1-675.
- Lebas D, Amici JM (2020) Introduction to tissue mobilizations. Flap principles, EMC-Dermatology.
- Qian Y, Li G, Zang H, Cao S, Liu Y, et al. (2017) A systematic review and meta-analysis of free-style flaps: Risk analysis of complications. Plast Reconstr Surg Glob Open 6(2).
© 2022 Jesús Nicolás,Larco Coloma. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






