- Submissions

Full Text
Surgical Medicine Open Access Journal
Investigation of the Efficacy of Aged Algan Hemostatic Agent in Experimental Femoral Artery Bleeding Model in Rats
Ali Kumandaş1* and Selin İbrişim2
1Department of Surgery, Turkey
2chool of Medicine, Turkey
*Corresponding author: Ali Kumandaş, Faculty of Veterinary Medicine, Department of Surgery, Kirikkale, Turkey
Submission: November 5, 2021Published: February 28, 2022

ISSN 2578-0379 Volume4 Issue5
Abstract
Effective and fast-acting hemorrhage stopper materials are still needed in the first intervention of trauma patients with large vessel injuries. In this study, we investigated the efficacy of aged Algan Hemostatic Agent® (AHA) in an experimental rat model with femoral artery hemorrhage. In this study, 21 male rats, 5-7 weeks old and weighing 200-250 grams, were used. Rats were randomly divided into three groups, 7 animals in each group. The femoral artery and vein were visualized and bleeding was initiated with an incision. Bleeding time was recorded. Those whose bleeding stopped in the first two minutes were evaluated as those whose bleeding stopped in the first four minutes. If bleeding still continued after the fourth minute, it was recorded as unsuccessful. In the study group, bleeding could be controlled in 5 rats in the first application and in two rats in the second application. In the control group animals, hemorrhagic shock findings were detected because the bleeding could not be controlled after femoral artery bleeding. The difference between the AHA and control groups was statistically significant (p=0.001). It is known that the main treatment for the control of large arterial hemorrhages is surgical hemorrhage control. However, products with practical applicability can be used in non-hospital areas. According to our research results, it was determined that the 4-year-old AHA product successfully maintained its astringent activity. According to the results of the study, it was concluded that the AHA hemostatic product can be used as a first-aid hemorrhage stopper in practice.
Keywords:Algan hemostatic agent; Bleeding; Femoral artery; Hemostazis; Rat
Abbreviations:AHA: Algan Hemostatic Agent®; TRAP: Thrombin-Receptor-Agonist-Peptide
Introduction
Early controling of bleeding is one of the critical first steps in the management of trauma patients. However, achieving this goal still remains a serious challenge in critical trauma patients. Providing hemostasis in the early period with an agent that can be used by the person or the first aider in external bleeding will reduce the rate of disability and death in these patients. These agents to be used should be easily portable and applicable, inexpensive, without side effects, and easy to obtain. The need for such an agent will increase in disaster situations and wars with large numbers of casualties. Because it is difficult to intervene in a large number of bleeding wounds, to suture their bleeding wounds, and sometimes there is no time and team to do this. Especially in some anatomical localization (head, axillary, femoral region) injuries hemostasis is difficult to achieve [1-4].
It is reported that there are alternative applications including local hemostatic agents to reduce bleeding and complications such as postoperative infection. These agents are used as topical agents such as collagen, gelatin, or cellulose-based products, fibrin sealants, and synthetic adhesives, in addition to traditional surgical techniques, especially in patients with coagulation disorders [5-9]. In addition to above there are plant based product which are used for this aim [10]. The Algan Hemostatic Agent is a herbal extract derived from a standardized blend of six different plants (Table 1) that has a CE medical devices certification (Certification number: EC Design-Examination Certificate 1783-MDD-216) [11-15]. In our study, we aimed to investigate the effects of aged Algan Hemostatic Agent (AHA) on hemostasis in an experimental rat model in femoral artery bleeding.
Table 1. Algan hemostatic agent formulation.

Materials and Methods
Before the study, animals were kept in quarantine for 15 days and after health checks, healthy animals were included in the study. In the study, 21 male rats, 5-7 weeks old and weighing 200-250 grams, were used. Rats were randomly divided into three groups, with 7 animals in each group. 1st group (New production Algan Sponge), 2nd group (4 years aged Algan Sponge) and 3rd group (control group). Shelter conditions were adjusted to room temperature 22°C (±3 °C), humidity between 30%-70%, 12 hours of light, and 12 hours of darkness. For feeding, ad-libutum commercial rodent pellet feed was provided with a normal laboratory diet and unlimited water. Before the surgical procedure the animals were administered xylazine hydrochloride (2-5mg/kg) and Ketamine hydrochloride (40-50mg/kg) for anesthesia. For analgesia, 0.05mg/ kg butorphanol was administered.
The right inguinal regions of the rats were shaved and wiped with Batticon. After cutting the skin and subcutaneous tissues, the femoral artery and vein were made visible. Femoral artery total incision was made. In the meantime, as soon as the bleeding started, the pressure was applied with gauze for 10 seconds. After the gauze was removed, 4 years aged AHA and saline-impregnated gas were applied to the bleeding site for 2 minutes. A 55g stainless steel mass was used for the pressure (Figure 1). Time was followed by running the stopwatch at the same time. After two minutes, this buffer and study materials were removed, and bleeding was controlled. If the bleeding has stopped, it is recorded as the bleeding has been controlled. If the bleeding did not stop, a second twominute application was made, and the test was continued. After the bleeding stopped or the experiment was terminated, the animal’s vital signs were evaluated. The rats were euthanized with CO2 at the earliest 10 minutes after the end of the experiment.
Histopathologic investigation
For histopathological examination, tissues were taken from the area where AHA was applied. Tissue specimens were routinely processed in the pathology laboratory and examined under a light microscope. These procedures were briefly carried out as follows: The tissue was followed by routine tissue fixation in neutral buffered formalin for a period of time, dehydrated in graded alcohols and embedded in paraffin. 5mm thick tissue sections were cut and stained with hematoxylin and eosin. Under the light microscope, necrosis and the presence of AHA residues were assessed.
Result
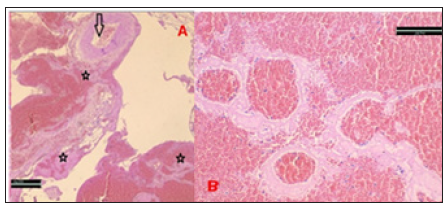
In the AHA groups, bleeding was controlled in 5 rats in the first two-minute application and in two rats in the second two-minute application (Figure 1). In the control group animals, hemorrhagic shock findings were detected because the bleeding could not be controlled after femoral artery bleeding (Table 2). Histopathological examination revealed vascular congestion and no residual AHA material. Tissue necrosis wasn’t observed (Figure 2).
Table 2.Bleeding stopping times in groups.
Figure 1: A: Local hemorrhage after femoral artery incision. B: Weight application. C: In the control group, after the clots are cleared, it is seen that there is no bleeding again. D: In the control group, bleeding continued after the application.

Figure 2: A: No AHA and thrombus are observed in the lümen of the vessels (arrow), in the perivascular area undamaged structures, erythrocyte and blood cell clustering around proteinous material are seen (stars) (H&E X40). B: After AHA application, erythrocytes and blood cells trapped in the proteinous network are seen in the extravascular area (H&EX200).
Discussion
This study demonstrated that 4-years aged AHA and AHA new pruduct are effective in controlling femoral artery bleeding. In addition results showed that 4-years aged AHA is as effective as AHA new pruduct. In a similar rat femoral artery study conducted in literature, hemostasis was achieved in the control group and the gelatin sponge group at a similar time, while hemostasis was achieved in a shorter time in the Thrombin-Receptor-Agonist- Peptide (TRAP) and Arista® groups [16]. In another study, while Ankaferd provided hemostasis in 4 rats in the first two minutes, it was able to provide hemostasis in 6 rats in the second application. In Chitosan, hemostasis was achieved in 3 rats in the first application, and in 6 rats in the second application, but hemostasis could not be achieved in one rat [17].
In our study, both the new product and the aged product provided hemostasis in 5 rats in the first 2 minutes, while in 2 rats it was able to provide hemostasis in the second application. Both the new and aged AHA 2 application has been successful in providing hemostasis in all rats. In another study while hemostasis was achieved in 260±16 seconds in the control group, in the crosslinked collagen sponge group hemostasis was achieved in 137±10 seconds [18]. In this study, after applying hemostatic drees to the damaged femoral artery area for 60 seconds, the hemostatic dress was removed and checked every 20 seconds until hemostasis was achieved. In another study, a massive hemorrhage model was created by cutting the femoral artery and vein, and the effectiveness of dextran-montmorillonite composite sponge and celox was investigated. In this study, dextran-montmorillonite composite sponge provided hemostasis in 53±4.2s (n=6) while it was 108.7±8.0 s and 128±8.0s respectively in the celox and standard gauze groups [19]. It is important that hemostatic agents do not cause side effects besides the hemostatic effect. Some hemostatic agents cause tissue damage by forming exothermic reactions [20].
In our current study, histopathological examination shows that the tissues taken from the AHA application area did not have tissue damage. Additionally, it has also been shown in previous studies that AHA does not cause tissue damage, contributes positively to wound healing and reduces intra-abdominal adhesion [11,12,21]. There are limited studies on the effectiveness of aged versions of hemostatic products. Homeostatic agents are mainly used to stop venous and small arterial bleeding, but in this study they were used in significant arterial bleeding and it was determined that it provided effective hemostasis even in large bleedings.
Although the effectiveness of existing products has been proven, there is no information on whether their effectiveness decreases after a certain shelf life. In this study, which aimed to determine the efficacy of AHA during its post-production shelf life, this hemostatic agent aged 4 years was still found to be effective. As a results various procedures such as direct compression, tourniquet, and clamp are used to stop bleeding. But these methods are not always successful. Homeostatic products are now manufactured to deal with severe bleeding due to trauma. Although AHA was shown to be an effective agent in this study, it would be more appropriate to conduct comparative studies with other widely used hemostatic agents which have been proven to be effective and to evaluate according to the results of their clinical use.
Conclusion
According to the experimental results of this study, it was determined that AHA, which was found to be an effective hemostatic agent in previous studies, effectively provides hemostasis when the aged product is used. It is thought that further experiments by evaluating the current study in comparison with different bleeding models and other available hemostatic agents will yield beneficial results.
Acknowledgment
The authors do not have any relationship with the commercial company of the product tested (AHA). The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Ethics Committee Approval
For this study, approval for animal experimentation was obtained from KU Animal Experiments Local Ethics Committee (Decision no 2018/08).
References
- Ersoy G, Kaynak F, Yilmaz O, Rodoplu U, Maltepe F, et al. (2007) Hemostatic effects of microporous polysaccharide hemosphere in a rat model with severe femoral artery bleeding. Advances in Therapy 24(3): 485-492.
- Ward KR, Tiba MH, Holbert WH, Blocher CR, Draucker GT, et al. (2007) Comparasion of a new hemostatic agent in a swine model of lethal extremity arterial hemorrhage. J Trauma 63(2): 276-284.
- Ersoy G, Kaynak MF, Yilmaz O, Rodoplu U, Maltepe F, et al. (2007) Hemostatic effects of microporous polysaccharide hemosphere in a rat model with severe femoral artery bleeding. Adv Ther 24(3): 485-492.
- Ward KR, Tiba MH, Holbert WH, Blocher CR, Draucker GT, et al. (2007) Comparison of a new hemostatic agent to current combat hemostatic agents in a swine model of lethal extremity arterial hemorrhage. J Trauma 63(2): 276-283.
- Akarsu C, Kalayci MU, Yavuz E, Ozkara S, Gökçek B, et al. (2011) Comparison of the hemostatic efficiency of ankaferd blood stopper and fibrin glue on a liver laceration model in rats. Turkish Journal of Trauma& Emergency Surgery 17(4): 308-312.
- Beyazit Y, Kurt M, Kekilli M, Goker H, Haznedaroglu IC (2010) Evaluation of hemostatic effects of ankaferd as an alternative medicine. Altern Med Rev 15(4): 329-336.
- Aktop S, Emekli-Alturfan E, Ozer C, Gonul O, Garip H, et al. (2014) effects of ankaferd blood stopper and celox on the tissue factor activities of warfarin-treated rats. Clin Appl Thromb Hemost 20(1): 16-21.
- Zhang YJ, Gao B, Liu XW (2015) Topical and effective hemostatic medicines in the battlefield. Int J Clin Exp Med 8(1): 10-19.
- Chan LW, Kim CH, Wang X, Pun SH, White NJ, et al. (2016) PolySTAT modified chitosan gauzes for improved hemostasis in external hemorrhage. Acta Biomater 31: 178-185.
- Huri E, Akgül KT, Yücel MÖ, Astarci HM , Üstün H, et al. (2011) The second step in vitro trial of Ankaferd® Bloodstopper®: Comparison with other hemostatic agents. Turk J Med Sci 41(1): 7-15.
- Midi A, Kumandaş A, Ekici H, Arda S, Karahan S, et al. (2018) Investigation of the effectiveness of algan hemostatic agent in renal venous bleeding model in rats. EJMI 2(3): 129-132.
- Midi A, Ekici H, Kumandas A, Durmus O, Bodic B, et al. (2019) Investigation of the effectiveness of algan hemostatic agent in bleeding control using an experimental partial splenectomy model in rats. Marmara Medical Journal 32(1): 27-32.
- Midi A, Kumandas A, Ekici H, Bayraktar F, Karapirli K, et al. (2019) Investigation of the efficacy of algan hemostatic agent in liver laceration model in rats. EJMO 3(1): 37-42.
- Midi A, Ozyurek HE, Karahan S, Ekici H, Kumandas A, et al. (2018) Investigation of efficacy of the plant based algan hemostatic agent in hepatectomy bleeding model in rats. EJMI 2(4): 195-201.
- Totuk ÖMG, Güzel ŞE, Ekici H, Kumandaş A, Aydıngöz SE, et al. (2020) Effects of algan hemostatic agent on bleeding time in a rat tail hemorrhage model. Turkish Journal of Trauma and Emergency Surgery 26(6): 853-858.
- Yang X, Liu W, Shi Y, Xi G, Wang M, et al. (2019) Peptide-immobilized starch/PEG sponge with rapid shape recovery and dual-function for both uncontrolled and noncompressible hemorrhage. Acta Biomater 99: 220-235.
- Abacıoğlu S, Aydin K, Büyükcam F, Kaya U, Işik B, et al. (2016) Comparison of the efficiencies of buffers containing ankaferd and chitosan on hemostasis in an experimental rat model with femoral artery bleeding. Turk J Haematol 33(1): 48-52.
- Sun L, Li B, Song W, Zhang K, Fan Y, et al. (2020) Comprehensive assessment of nile tilapia skin collagen sponges as hemostatic dressings. Mater Sci Eng C Mater Biol Appl 109: 110532.
- Liu C, Liu C, Yu S, Wang N, Yao W, et al. (2020) Efficient antibacterial dextran-montmorillonite composite sponge for rapid hemostasis with wound healing. Int J Biol Macromol 160: 1130-1143.
- Arnaud F, Tomori T, Carr W, McKeague A, Teranishi K, et al. (2008) Exothermic reaction in zeolite hemostatic dressings: QuikClot ACS and ACS+. Annals of Biomedical Engineering 36(10): 1708-1713.
- Aksoy H, Sener A, Akakin D, Sen A, Akpınar OB, et al. (2020) The Effect of Algan Hemostatic Agent (AHA) on wound healing. Clinical and Experimental Health Sciences 10(3): 279-284.
© 2022 Ali Kumandaş. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






