- Submissions

Full Text
Surgical Medicine Open Access Journal
For the Right Hemiliver Graft May Need Tissue Expander After Living Donor Liver Transplantation
Batsaikhan BE2, Sergelen O1, Batsaikhan B1*, Bat IB1, Ganzorig B1, Erdene S2 and Urnult G1
1Surgical Department, The First Central Hospital of Mongolia, Mongolia
2Department of Surgery, Mongolian National University of Medical Science, Mongolia
*Corresponding author: Batsaikhan B, Surgical Department, The First Central Hospital of Mongolia, Mongolia
Submission: January 27, 2021Published: March 01, 2021

ISSN 2581-0379 Volume4 Issue2
Abstract
Introduction: Hepatic venous outflow is an important for the graft survival in living donor liver transplantation. Hepatic venous outflow obstruction generates liver failure, which may influenced due to graft malposition, which unsized upper part of abdomen between donor and recipient. The number of Liver Transplantation (LT) increases last few years, which is related with populating the high surgical technology in developing country like in Mongolia. The main reason of LT is a hepatitis B virus-related liver cirrhosis in Mongolia. Liver transplantation had started since 2011 under the supporting of Professor Sung Gyu Lee from hepato-biliary surgery and liver transplantation of ASAN Medical Center. Veno-Occlusive Disease (VOD), Budd-Chiari Syndrome (BCS), and Congestive Hepatopathy (CH), all of which results in hepatic venous outflow obstruction. The early Hepatic Venous Outflow Obstruction (HVOO) is a rare, however that could raise a serious complication as a graft failure and eventual lose. We report a case of early HVOO, which may result of size mismatch of abdominal cavity. The size mismatch of abdominal cavity may produce kinking syndrome after transplantation of right lobe, which reveals the HVOO without anastomosis complication. Methods: A 38-year-old male patient with liver cirrhosis due to HBV, HDV, HCC in S8 of the liver (CTP-B, MELD-18). On the first postoperative day the patient developed impairment of the liver function. Doppler ultrasound (US) showed the different speed of RHV preanastomosis and postanastomosis field. This was diagnosed acute liver failure due to veno-oclusive disease, after that started intensive therapy.
Result: Kinking or twisting of the venous anastomosis is related with anatomical mismatch between the graft and the recipient abdomen, even though transplanted the right hemiliver graft. HVOO results acute cellular rejection, which treated by pulse therapy. However, it should be managed by surgically, put the tissue expander.
Conclusion: Doppler ultrasonography is one of the best choices to evaluate postoperative vascular complications in liver transplantation. The right hemiliver graft needs tissue expander for mismatching between the graft and recipient abdomen.
Introduction
Living donor liver transplantation has become the treatment of choice for patients
with end-stage acute or chronic hepatic disease in developing country like Mongolia. Still
vascular complication is one of the serious complications of liver transplantation, which leads
postoperation graft failure [1]. Vascular complication was ranging from 8% to 15% among
transplantation centers after liver transplantation. The rate could be high in split LT or LDLT [2-
5]. Size mismatch of abdominal cavity is a rare for living donor liver transplantation, however
it offers numerous disadvantages after transplantation. A few studies have suggested that
mismatch for size of liver, but there had not reported the size mismatch of abdominal cavity,
which directs Budd-Chiari Syndrome (BCS). Although studies from India mentioned that the
most common site of obstruction is combined hepatic vein and inferior vein cava (56-58%),
only IVC obstruction was reported in 14-50% cases of HVOO [6-9].
Researchers reported incidences of Hepatic Venous Outflow Obstruction (HVOO) after
living donor liver transplantation are 3.9-16.6% [10-13]. In LDLT recipients, HVOO is becoming
a reason of liver function deterioration, graft failure, or even death resulting from the small size of the graft [14,15]. Right hepatic vein obtained insufficient
for reconstruction during the LDLT, which provides development
of HVOO [13,16]. HVOO occurs in the early postoperative period
(≤28 days) are caused by technical factors such as a tight suture
line, donor-recipient size discrepancy, kinking of a redundant
hepatic vein, and caval compression from a large graft [12,17].
Clinical symptoms of HVOO are non-specific, however could be
abnormal liver function, hepatomegaly, ascites, pleural effusion,
and lower-extremity edema [10,13,18]. BCS is an uncommon
disorder characterized by obstruction of hepatic venous outflow
and considered primary or secondary depending on the origin of
the obstructive lesion. The obstruction cause could be thrombotic
or non-thrombotic, that size mismatch of abdomen is a reason of
kinking syndrome.
Case
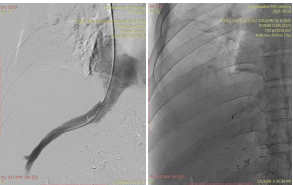
The patient is 38 years old, man diagnosed HBV+HDV related LC, HCC on S8, CTP-B, MELD score 18. He underwent living donor liver transplantation operation using a right lobe graft from younger sister. The recipient’s body weight is an 84kg, height 172cm, body mass index 28.4, body surface area 2m2. The donor’s body weight is 58kg, height 153cm, body mass index 24.8, body surface area 1.6m2. The operation time was continued 15hours 34 minutes, the cold is 85 minutes and warm ischemic is 55 minutes. There were directly increased some laboratory tests postoperation day 1, serum total bilirubin 3.77mg/dl, alanine transaminase (ALT) 657.32 (IU/L), Aspartate Transaminase (AST) 458.89 (IU/L), Gamma Glutamyl Transpeptinase (GGT) 21.42 (IU/L), and LD 585.87 (IU/L). POD 2 showed the increased serum total bilirubin 3.81mg/dl, Alanine Transaminase (ALT) 2866.32 (IU/L), Aspartate Transaminase (AST) 2306.3(IU/L), Gamma Glutamyl Transpeptinase (GGT) 37.14 (IU/L), and LD 1894.77 (IU/L). Ultrasonography showed different outflow speed in the anastomosis area of right hepatic vein (38.58cm/s) and in the middle area of RHV (68.04cm/s), which expected the kinking syndrome in POD2 (Figure 1). CT with contrast showed right hepatic vein stenosis (Figure2A & 2B). Venography showed pre-stenotic pressure of RHV-20mmHg, and IVC-7mmHg (Figure 3). After stenting the outflow was reestablished and intra hepatic venous pressure was decreased to 7mmHg, which was shown in Figure 4A & 4B. Hepatic venous pressure gradient was decreased from 13mmHg to 1-2mmHg.
Figure 1.

Figure 2A & 2B.
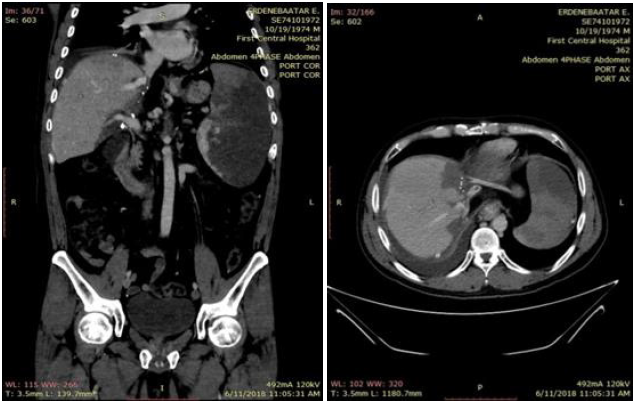
Figure 3.

Figure 4A & 4B.
Discussion
Doppler Ultrasound (US) is widely used for the controlling
patients and checking inflow or outflow of liver after
transplantation. Hwang et al. reported that Doppler ultrasound
for the diagnosis of main hepatic vein tributary obstruction and
inferior RHV obstruction was more sensitive and accurate than
CT (97% vs 39%), however less specific (67% vs 100%) [19].
Computed Tomography (CT) is commonly used in controversial
clinical patients. CT’s sensitive is a (97% vs. 87%) and specificity
(86% vs. 68%), that results are better than Doppler US, which could
suggest venous congestion [20]. Pressure gradients have a main
role in diagnosis of HVOO after liver transplantation, that across the
stenosis between the distal hepatic vein and the right atrium that
was > 5mmHg [21]. A pressure gradient is a >5-6mmHg, which is
commonly accepted as a threshold of HVOO.
Radiological dilatation and stenting are a successful
management for HVOO for the majority patients. The incidence of
HVOO after liver transplantation varies from 5% to 9.5%, from that
whole liver transplantation has low percentage of the incidence.
The highest percentage of HVOO had faced with pediatric and
living donor, split liver transplantation. Early causes of HVOO are
twisting or kinking at the anastomosis as a result of rotation of the
graft because of the lack of adhesions in the early post-operative
period. There are other early causes include a tight anastomosis,
thrombosis and compression of anastomosis as a result of graft
swelling or haematoma. The late causes include neointimal
hyperplasia and fibrosis structuring at the anastomosis [22].
Conclusion
Doppler ultrasonography is one of the best choices to evaluate postoperative vascular complications in liver transplantation. The right hemiliver graft needs tissue expander for mismatching between the graft and recipient abdomen.
References
- Duffy JP, Hong JC, Farmer DG (2009) Vascular complications of orthotopic liver transplantation: Experience in more than 4,200 patients. J Am Coll Surg 208(5): 896-903.
- Piardi T, Lhuaire M, Bruno O (2016) Vascular complications following liver transplantation: A literature review of advances in 2015. World J Hepatol 8(1): 36-57.
- Kenari SKH, Zimmerman A, Eslami M (2014) Current state of art management for vascular complications after liver transplantation. Middle East J Dig Dis 6(3): 121-130.
- Khalaf H (2010) Vascular complications after deceased and living donor liver transplantation: A single-center experience. Transplant Proc 42(3): 865-870.
- Sindhi R, Rosendale J, Mundy D (1999) Impact of segmental grafts on pediatric liver transplantation-a review of the United Network for organ sharing scientific registry data (1990-1996). J Pediatr Surg 34(1): 107-110.
- Dilawari JB, Bambery P, Chawla Y (1994) Hepatic outflow obstruction (Budd Chiari syndrome). Experience with 177 patients and a review of the literature. Medicine 73(1): 21-36.
- Singh V, Sinha SK, Nain CK (2000) Budd-Chiari syndrome: Our experience of 71 patients. J Gastroenterol Hepatol 15(5): 550-554.
- Amarapurkar DN, Punamiya SJ, Patel ND (2008) Changing spectrum of Budd-Chairi syndrome in India with special reference to non-surgical treatment. World J Gastroenterol 14(2): 278-285.
- De BK, De KK, Sen S (2000) Etiology based prevalence of budd-chiari syndrome in eastern India. J Assoc Physicians India 48: 800-803.
- Egawa H, Tanaka K, Uemoto S (1993) Relief of hepatic vein stenosis by balloon angioplasty after living-related donor liver transplantation. Clin Transplant 7(4): 306-311.
- Emond J, Heffron T, Whitington P, Broelsch CE (1993) Reconstruction of the hepatic vein in reduced size hepatic transplantation. Surg Gynecol Obstet 176(1): 11-17.
- Someda H, Moriyasu F, Fujimoto M, Hamato N, Nabeshima M, et al. (1995) Vascular complications in living related liver transplantation detected with intraoperative and postoperative Doppler US. J Hepatol 22(6): 623-632.
- Egawa H, Inomata Y, Uemoto S, K Asonuma, T Kiuchi, et al. (1997) Hepatic vein reconstruction in 152 living-related donor liver transplantation patients. Surgery 121(3): 250-257.
- Lee S, Park K, Hwang S, Ki HK, Chul SA, et al. (2003) Anterior segment congestion of a right liver lobe graft in living-donor liver transplantation and strategy to prevent congestion. J Hepatobiliary Pancreat Surg 10(1): 16-25.
- Fan ST, Lo CM, Liu CL, Boon HY, John W, et al. (2003) Determinants of hospital mortality of adult recipients of right lobe live donor liver transplantation. Ann Surg 238(6): 864-870.
- Takayama T, Makuuchi M, Kawarasaki H, S Kawasaki, H Matsunami, et al. (1994) Venacavoplasty to overcome outflow block in living related liver transplantation. Transplantation 58(1):116-118.
- Chen YS, Chen CL, Liu PP, Wang CC, Chian YC, et al. (1998) Successful treatment of hepatic vein thrombosis following reduced-size liver transplantation. Transplant Proct 30(7): 3203-3204.
- Zajko AB, Claus D, Clapuyt P, Esquivel CO, Moulin D, et al. (1989) Obstruction to hepatic venous drainage after liver transplantation: Treatment with ballon angioplasty. Radiology 170(3): 763-765.
- Hwang HJ, Kim KW, Jeong WK, So YK, Gi WS, et al. (2009) Hepatic outflow obstruction at middle hepatic vein tributaries or inferior right hepatic veins after living donor liver transplantation with modified right lobe graft: Comparison of CT and Doppler ultrasound. AJR Am J Roentgenol 193(3): 745-751.
- Hwang HJ, Kim KW, Jeong WK, Gi WS, Gi YK, et al. (2009) Right hepatic vein stenosis at anastomosis in patients after living donor liver transplantation: Optimal Doppler US venous pulsatility index and CT criteria-receiver operating characteristic analysis. Radiology 253: 543-551.
- Jang JY, Jeon UB, Park JH, Tae UK, Jun WL, et al. (2017) Efficacy and patency of primary stenting for hepatic venous outflow obstruction after living donor liver transplantation. Acta Radiol 58(1): 34-40.
- Arudchelvam J, Bartlett A, McCall J, Peter J, Edward G, et al. (2017) Hepatic venous outflow obstruction in piggyback liver transplantation: Single centre experience. ANZ J Surg 87(3): 182-185.
© 2021 Talar VA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






