- Submissions

Full Text
Surgical Medicine Open Access Journal
Bortezomib-Induced Blepharitis: A Case Report
Ahmad Khalil1, Pamela Sfeir1, Antoine Abi Abboud2, Kamal AlZahran1*, Elise Slim3 and Evelyne Helou4
1Department of Hematology-Oncology, Lebanon
2Department of Gastro-Enterology, Lebanon
3Department of Ophthalmology, Lebanon
4Department of Hematology-Oncology, Lebanon
*Corresponding author: Kamal AlZahran, Department of Hematology-Oncology, Middle East Institute of Health (MEIH), Beirut, Lebanon
Submission: January 16, 2020;Published: January 27, 2020

ISSN 2578-0379 Volume3 Issue3
Abstract
Introduction: Bortezomib is a proteasome inhibitor approved for the treatment of multiple myeloma and has known manageable toxicities. Blepharitis is an inflammatory condition of the eyelid that leads to formation of chalazia both causing visual field disturbance. Bortezomib induced blepharitis has not been well reported in the literature.
Case: we present a case of 76-year-old lady with multiple myeloma who developed bortezomibinduced blepharitis and chalazia. Patient was successfully treated after topical ocular therapy, systemic antibiotherapy and omission of bortezomib.
Keywords: Multiple myeloma; Blepharitis; Chalazia; Bortezomib
Introduction
Multiple myeloma is characterized by a neoplastic proliferation of plasma cells in the bone marrow producing a monoclonal immunoglobulin and resulting in extensive skeletal destruction. One of the used treatment regimens for MM being the VCD protocol (Velcade® or Bortezomib, Cyclophosphamide and Dexamethasone) [1,2]. Bortezomib is a proteasome inhibitor that acts by disrupting the cell cycle and inducing apoptosis, usually tolerated in the outpatient settings with manageable toxicities, the most common ones being peripheral neuropathy and thrombocytopenia [1,2]. Blepharitis is an inflammatory condition of the eyelid margin causing ocular and visual discomfort leading to formation of chalazia (lipogranulomatous lesions) [3,4]. It is classified as anterior blepharitis when it involves the eyelid skin and the follicules and posterior when it involves the Meibomian glands; it is usually associated with staphylococcus aureus colonization, infestation with parasites, Meibomian gland dysfunction, rosacea, systemic use of docetaxel [3,4]. Bortezomib has been associated with ocular complications but it has not been well characterized. We herein present a case of Bortezomib-induced blepharitis that has been successfully treated.
Case Report
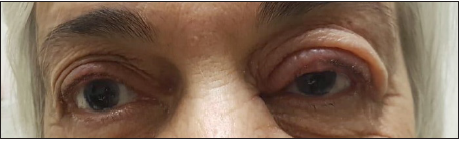
A 76-year-old lady diagnosed with multiple myeloma IgG kappa light chain stage III in August 2018 after she was found to have anemia, elevated creatinine (1.5g/dL) and a serum protein electrophoresis showing 17.5g/dL of M protein (monoclonal peak). Patient was started on VCD protocol (weekly based) and zoledronic acid for malignant hypercalcemia. After completion of two treatment cycles, in October 2018, patient was found to have a left painful itchy red eye. Ophthalmologic exam showed edema and redness of the left upper eyelid with preserved visual fields, no diplopia, no discharge. Fundoscopy was normal. There was no evidence of viral or upper respiratory tract infection. Patient then started on doxycycline (daily dose of 100mg) and local framycetin (Frakidex®) to apply four times daily on the affected eye while continuing her chemotherapy treatment. She then developed 2 new upper eyelid chalazia, while receiving her blepharitis treatment. Significant improvement was observed when combining blepharitis treatment with bortezomib cessation. In November 2018, patient had a right trans-cervical hip fracture, no anemia, stable chronic kidney disease and negative PET scan so chemotherapy was stopped. Follow up ophthalmologic exam after 3 months showed a complete remission of the blepharitis (Figure 1).
Figure 1: Left red eye and chalazia in left upper eyelid.
Gene Expression
We reported a case of bortezomib induced blepharitis and
chalazia successfully treated after topical therapy and systemic
antibiotherapy (doxycycline) associated with omission of
chemotherapy. A literature review done showed 28 cases of
bortezomib induced blepharitis reported. Most recently, the
association between bortezomib and chalazia was classified as a
“possible” adverse drug reaction (ADR) based on the World Health
Organization’s definition of ADRs and an analysis of both case
reports in the literature as well as reports submitted to the National
Registry of Drug-Induced Ocular Side Effects [5,6].
In a study conducted by Sklar et al. [7]16 patients treated
with bortezomib developed ocular complications after 3.4 months
in average. 100% of blepharitis and 44% of chalazia resolved
completely after ocular therapy alone whereas 80% of chalazia and
60% of blepharitis resolved after ocular therapy and chemotherapy
omission with longer periods needed for remission of blepharitis
(117 day) versus chalazia (74 days) [7]. In our case, after a
treatment with oral doxycycline and topical framycetin, blepharitis
and chalazia didn’t improve. It only resolved after 30 days when
bortezomib was omitted.
Grob SR et al. [8]. reported a case series in which 6 patients
developed chalazia within 3.3 months of bortezomib therapy
beginning. All conservative measures (including warm compresses
and lid hygiene) failed. Four patients underwent incision and
curettage and 5 of 6 patients had bortezomib suspended or
discontinued due to eye discomfort [8]. Ince bortezomib inhibits
the ubiquitin proteasome pathway, proapoptotic molecules
accumulates leading to apoptosis of neoplastic cells. In parallel,
bortezomib influence other inflammatory pathways including
NF-kB, JAK/STAT, and MAP kinase, promoting release of proinflammatory
cytokines. Interference of all these pathways in the
eyelids contributes in inflammatory flares causing blepharitis and/
or chalazia [9]. So, the pathogenesis of blepharitis and chalazia in
proteasome inhibitor treated patients is postulated to be related to
inflammation [9-11]. This hypothesis is further proved by the fact
that blepharitis resolved with local anti-inflammatory treatment
and systemic antibiotic.
Studies postulated that a baseline eye examination is
encouraged before starting proteasome inhibitor therapy [7].
Treatment of chalazia/blepharitis induced by bortezomib should
be started with conservative measures (hot compresses in
combination with at least 1 topical antibiotic and/or steroid drop,
and possible oral antibiotics as well). If eyelid complications persist
after this treatment, omission of bortezomib should be considered
with a switch to alternative proteasome inhibitors (carfilzomib or
ixazomib). Oral doxycycline can be added if no improvement. After
bortezomib omission, if eyelid complications resolve, are challenge
can be considered. However, if eyelid complications recur upon
bortezomib re challenge, the drug should be discontinued again [7].
In our case, patient improved after local therapy, oral doxycycline
and omission of bortezomib. No re-challenge or switch to another
proteasome inhibitor was done in our case because of the patient’s
fracture and stable disease.
Conclusion
We herein presented a case of bortezomib-induced blepharitis and chalazia that has been successfully treated after ocular therapy, systemic antibiotherapy and omission of the bortezomib. So, awareness, early detection, and prompt management of proteasome inhibitors eyelid complications, (although rare) will help improve the quality of life of patients.
References
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, et al. (2003) A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 348(26): 2609-2617.
- Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, et al. (2005) Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol 129(6): 776-783.
- Kanski JJ (1994) Clinical ophthalmology: A systematic approach. (5th edn), Butterworth-Heinemann Ltd, UK.
- Ballen PH (1964) Inflammations of the lid. Int Ophthalmol Clin 4: 5-20.
- Fraunfelder FW, Yang HK (2016) Association between bortezomib therapy and eyelid chalazia. JAMA Ophthalmol 134(1): 88-90.
- Veys MC, Delforge M, Mombaerts I (2016) Treatment with doxycycline for severe bortezomib-associated blepharitis. Clin Lymphoma Myeloma Leuk 16(7): e109-e112.
- Sklar AB, Gervasio KA, Leng S, Ghosh A, Chari A, et al. (2019) Management and outcomes of proteasome inhibitor associated chalazia and blepharitis: A case series. BMC Ophthalmology 19(1): 110.
- Grob SR, Jakobiec FA, Rashid A, Yoon MK (2014) Chalazia associated with bortezomib therapy for multiple myeloma. Ophthalmology 121(9): 1845-1847.
- Sunwoo JB, Chen Z, Dong G, Yeh N, Crowl BC, et al. (2001) Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res 7(5): 1419-1428.
- Yun C, Mukhi N, Kremer V, Shinder R, Verma V, et al. (2015) Chalazia development in multiple myeloma: A new complication associated with bortezomib therapy. Hematol Rep 7(2): 5729.
- Mohamed R, Massa H, Schwarz C, Grandjean HN, Samii K (2015) Bortezomib induced multiple chalazia: A case report. Case Reports in Clinical Medicine 4(1): 32-35.
© 2020 Kamal AlZahran. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






