- Submissions

Full Text
Significances of Bioengineering & Biosciences
Prenatal Exposure to Cannabinoids Decreases the Expression of Polysialylated-Neural Cell Adhesion Molecule (PSA-NCAM)
Priyanka D Pinky1, Jenna Bloemer2, Vishnu Suppiramaniam1,3,4* and Miranda N Reed1,4*
1Department of Drug Discovery and Development, Auburn University, USA
2Department of Pharmaceutical Sciences, College of Pharmacy, Larkin University, USA
3Department of Molecular and Cellular Biology, College of Science and Mathematics, Kennesaw State University, Georgia
4Center for Neuroscience Initiative, Auburn University, USA
*Corresponding author:Vishnu Suppiramaniam, Department of Drug Discovery and Development, Auburn University, USA, Department of Molecular and Cellular Biology, College of Science and Mathematics, Kennesaw State University, Georgia, Center for Neuroscience Initiative, Auburn University, USA
Miranda N Reed, Department of Drug Discovery and Development, Auburn University, USA, Center for Neuroscience Initiative, Auburn University, USA
Submission: March 27, 2024; Published: April 03, 2024

ISSN 2637-8078Volume6 Issue5
Abstract
The use of cannabis during pregnancy has continued to rise in recent years despite recent studies in humans showing that prenatal exposure can elicit developmental delays and long-lasting cognitive deficits. Using a rodent model, this preclinical study investigates the consequences of prenatal cannabis exposure to Delta-9-tetrahydrocannabinol (THC) on offspring development, including the day of fur development and eye-opening, spontaneous righting reflex on postnatal days 5, 8 or 11 and rate of bodyweight gain. We also examined the protein expression of Neural Cell Adhesion Molecule (NCAM) and Polysialylated-NCAM (PSA-NCAM), which is required for neurogenesis, neuronal pathfinding, learning and memory and has been shown in our previous studies to be linked to synaptic plasticity deficits in offspring exposed to a cannabinoid agonist prenatally. THC- exposed offspring did not differ on the day of fur development and eye-opening. Spontaneous right reflex was significantly shorter on postnatal day 5 but returned to that of vehicle-exposed pups on postnatal days 8 and 11. Bodyweight was slightly decreased for THC-exposed offspring during early development but did not differ from vehicle-exposed offspring by postnatal day 45. THC-exposed offspring exhibited reduced hippocampal protein expression of PSA-NCAM during adolescence. These findings suggest prenatal exposure to THC can have long-term detrimental effects on the expression of PSA attached to NCAM, which is developmentally regulated and may provide one possible mechanism for the cognitive deficits associated with prenatal cannabinoid exposure.
Keywords: Prenatal; Cannabinoid; Developmental; Glutamate; Marijuana; Adolescence; Cannabis
Abbreviations: THC: Delta-9-tetrahydrocannabinol; NCAM: Neural Cell Adhesion Molecule; PSA-NCAM: Polysialylated- NCAM; PCE: Prenatal Cannabinoid Exposure; LTP: Long-Term Potentiation; GD: Gestational Day; PND: Postnatal Day.
Introduction
Cannabis use during pregnancy has increased dramatically in the past 10 years [1]. The relaxation of state-level marijuana policies is expected to result in even greater neonate levels [2], particularly as the perception that marijuana is safe for use during pregnancy has increased [3]. Despite this, there is a relative paucity of literature regarding the mechanisms mediating the cognitive deficits resulting from Prenatal Cannabinoid Exposure (PCE) [4,5]. This is particularly concerning given the high density of cannabinoid CB1 receptors in brain regions devoted to higher cognitive function, including the hippocampus and prefrontal cortex [6,7]. Moreover, CB1 receptors emerge early during prenatal development and are functionally coupled to signal transduction mechanisms from early prenatal stages in both rodents [8] and humans [9]. Given that CB1 receptors play an important role in CNS development, affecting synaptogenesis, proliferation and migration of neuronal cells, functional synaptic organization and signal transduction (as reviewed in [10]), persistent cognitive deficits after PCE are not surprising. Furthermore, the prenatal brain is particularly sensitive to maternal drug use, making the fetal brain vulnerable during ongoing brain development. Yet, mechanisms for these alterations have not been fully elucidated.
NCAM is a transmembrane glycoprotein important in several neuronal processes, including neurite outgrowth, cell migration and synaptogenesis. PSA is attached to the extracellular domain of NCAM, which greatly affects NCAM function [11]. PSA is added to the extracellular domain of NCAM by two polysialyltransferases: ST8SIA2 and ST8SIA4. NCAM is the major carrier for PSA. Therefore, NCAM knockout animals are also PSA deficient. PSANCAM plays an important role in hippocampal synaptic plasticity and the removal of PSA by endosialidase- N or knockout of ST8SIA4 leads to impairments in Schaffer collateral Long-Term Potentiation (LTP) and impaired memory [12,13]. We have previously shown that prenatal exposure to the cannabinoid agonist WIN55,21-2 reduces the protein levels of both NCAM and PSA-NCAM during the adolescence period. Moreover, administration of exogenous PSA rescues the deficits in basal synaptic transmission and long-term potentiation in WIN55,21-2-exposed rats [14]. In the current study, we aimed to determine whether prenatal THC exposure altered developmental milestones, including the time to fur development and the first eye-opening. We also sought to determine whether the spontaneous righting reflexes and body weights of exposed pups would differ. Finally, we examined NCAM and PSA-NCAM to determine whether prenatal THC exposure would produce similar deficits as that observed after prenatal WIN55,21-2 exposure.
Results
Patient presentation
To assess the effects of prenatal THC exposure, timed pregnant Sprague-Dawley rats were purchased from Envigo laboratories.
Pregnant rats received a daily dose of THC (5mg/kg) orally administered through a buccopharyngeal cannula from Gestational Day (GD) 5 to Postnatal Day (PND) 9. THC (Cayman) was dissolved in sesame oil (vehicle), as previously described [15]. Control pregnant rats received the same volume (0.2mL) of vehicle. Male offspring were examined. All procedures were carried out in accordance with NIH guidelines and approved by the Auburn University Animal Care and Use Committee (IACUC).
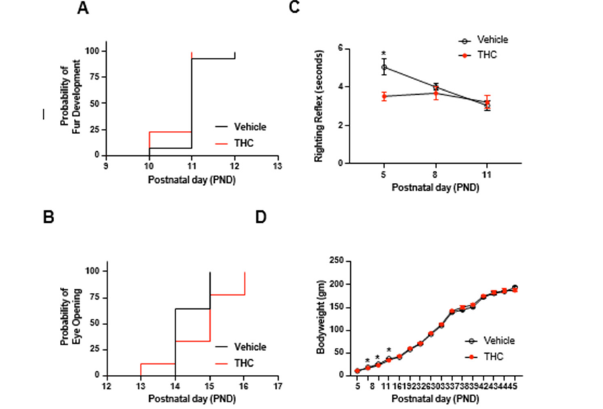
The median day to the first signs of fur development was PND 11 for both groups, and there was no significant difference between the groups (Mantel-Cox log-rank test, Chi square=1.577, p=.2092; Figure 1A). The time to eye opening was tracked daily and scored if at least one eyelid was open [16]. Though the median time to eye opening was PND 14 for vehicle-treated mice and PND 15 for THCtreated mice, this difference was not significantly different between the groups (Mantel-Cox log-rank test, Chi square=2.623, p=.1054; Figure 1B). Spontaneous righting reflex was assessed on PND 5, 8 and 11 by placing an unrestrained pup on its back (supine position) and measuring the time it takes for the animal to right itself with all four limbs on the ground [17]. THC-exposed pups exhibited a significantly shorter righting reflex on PND 5 but did not differ from vehicle-exposed pups on PND 8 and 11 (Mixed-effects analysis, Day*Group: F (2,61) =3.695, =.0306; Figure 1C). Bodyweights were assessed from PND 5-45 and were found to differ slightly as a function of the day (Mixed-effects analysis, Day*Group: F (15,315) =1.953, =.0182; Figure 1D), such that THC-exposed offspring weighed slightly less on PND 8, 11 and 16. Thus, though there were some differences in the initial postnatal days for righting reflex and body weight, these differences diminished as animals aged.
Figure 1:Effects of prenatal exposure to THC on development. (A) Survival plot to the day of 4irst signs of fur development. (B) Survival plot to the day of 4irst eye-opening. (C) The latency in seconds to spontaneous righting re4lex. (D) Bodyweights as a function of time. *p<.05, n=14 for vehicles and n= 9 for THC. Mean SEM.
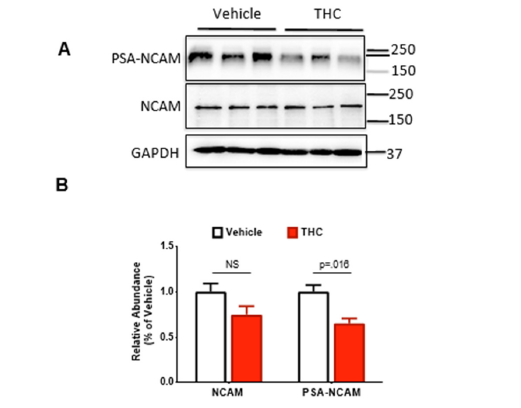
We have previously shown that prenatal exposure to the cannabinoid agonist WIN55,212-2 reduces the protein levels of both NCAM and PSA-NCAM during the adolescence period (PND 65) and that that restoration of PSA in the form of colominic acid can restore the deficits in basal synaptic transmission and long-term potentiation observed in rats prenatally exposed to WIN55,212-2 [14]. Here, we sought to determine whether prenatal exposure to THC would produce similar reductions in NCAM and PSA-NCAM during adolescence (PND 65). To do this, rats were euthanized and hippocampi dissected for immunoblotting, as previously described [14]. We observed no differences in NCAM, though PSA-NCAM was significantly decreased in THC-exposed offspring (t-test, p=.016; Figure 2A, B).
Figure 2:Effects of prenatal exposure on levels of THC on polysialylated-neural cell adhesion molecule (PSA-NCAM). (A) Representative western blot images for NCAM (EMD Millipore; 5032), PSA-NCAM (gift; see acknowledgments) and GAPDH (EMD Millipore; CB 1001) levels in the hippocampus. (B) Quanti4ication of western blot for NCAM and PSANCAM relative to GAPDH. Symbols represent means ± SEM. n=3/group.
Discussion
The dose of THC (5mg/kg) used in the present study has been
found to correspond to moderate cannabis exposure in humans
after correction for differences in body surface area [18]. We chose
a moderate dose because:
A. The potency in confiscated marijuana as measured by
THC content is approximately 300% higher than it was in the
1980s and this continues to rise [19].
B. The amount of marijuana consumed has also increased, in
part due to the rising popularity of blunts compared to joints or
pipes [20] and
C. Relaxation of marijuana policies in Colorado were
associated with a 10.4% increase in maternal marijuana use
and a 69% increase in neonate levels just two years after the
policy change (2012 vs. 2014) [2].
Therefore, we chose a moderate dose of cannabinoid to reflect the increasing potency and level of marijuana to which pregnant women are likely to be exposed. In addition, this dose is not associated with maternal or fetal abnormalities, including no alterations in maternal weight, fetal weight, litter size, gestation time or pup mortality [18,21,22]. Treatment was initiated at gestational day 5 as some data indicates that earlier administration can lead to spontaneous abortion [23]. We chose to treat during the postnatal period because the third trimester in humans corresponds to an early postnatal period in rats. Estimates of the PND in rats, which corresponds to the day of birth in humans, range from PND 7–21. This treatment regimen has also been validated in other studies of prenatal THC exposure [18,21,22]. Rats reach puberty at an average age of 50 days after birth (PND 50) and adolescence continues until approximately PND 65 [24]. We have opted to examine the consequences of THC on the adolescent period because most studies examining the consequences of prenatal exposure in humans have focused on this period, thereby allowing for comparison of our results to those obtained in humans. For example, observational human studies have demonstrated prenatal cannabinoid exposure results in cognitive impairments, including impairments in memory, analysis and attention, during the adolescent period [23,25]. Moreover, adolescent success is highly predictive of adulthood outcomes [26,27], so deficits during this period are likely to produce long-lasting consequences even if neurological alterations associated with prenatal exposure do not persist into adulthood.
We found prenatal THC exposure had minimum effect on development, as assessed by fur development, eye-opening, spontaneous righting reflex and body weight, similar to our prior studies with prenatal exposure to WIN55,212-2 [14]. A prior study examining the effects of prenatal THC via a vaporizer did observe more prolonged effects on body weight than that observed here, with vaporizer THC exposure causing a reduction in body weight up to PND 30 [28]. The human data is more mixed when it comes to birth weight, as some studies have reported increases though most have reported decreases (as reviewed in [29]). As noted in the review, few studies adjusted for known confounders of birth weight, such as maternal pre-pregnancy body mass index or gestational weight gain, and none adjusted for maternal diet in pregnancy. Unfortunately, few studies have examined the association between prenatal exposure to cannabis and postnatal growth, with only one human study showing that co-exposure to tobacco and cannabis was associated with rapid BMI growth from birth through midchildhood, though the effects of cannabis could not be isolated from those of tobacco [30]. Thus, whether prenatal THC exposure causes long-lasting changes in body weight remains unclear and may depend upon the route of administration or whether there is co-exposure to other drugs.
We did not observe differences in the time to first eye-opening, as previously shown following prenatal exposure to THC via a vaporizer [28]. To our knowledge, other studies have not examined the time to fur development after prenatal THC. Though the righting reflex has been shown to be altered when cannabis is given acutely at exceptionally high doses [31] or with other drugs known to affect the righting reflex (e.g., hexobarbital or pentobarbital [32,33], we did not find prior studies examining the righting reflex after prenatal exposure to THC. Prenatal THC is associated with memory deficits in both preclinical models and humans, as reviewed in [34]. As NCAM and PSA-NCAM are implicated in the maintenance of synaptic plasticity and memory [12,35], we previously hypothesized that the memory deficits observed following prenatal exposure to cannabinoids might be due to deficits in NCAM or PSA-NCAM. This hypothesis was supported by our findings that exogenous application of bacterially produced PSA rescued LTP deficits in offspring prenatally exposed to WIN55,212-2 [14], suggesting that PSA-NCAM represents a potential therapeutic target for the treatment of synaptic and cognitive deficits associated with PCE. In the current study, we observed a reduction in NCAM, though not statistically significant and a significant reduction in PSA-NCAM, suggesting THC may cause memory deficits through a reduction in PSA-NCAM. The differences in NCAM observed between the current study and our prior research [14] might stem from differences in drug potency. In our previous investigation, we administered a 2mg/kg dosage of WIN55,212-2. Given that WIN55,212-2 is estimated to be 3-10 times more potent than THC depending on the measured outcome [36,37], it’s plausible that the dosage utilized was more potent compared to the 5mg/kg of THC employed in the present study.
Conclusion
In summary, our study delved into the impact of prenatal THC exposure on developmental milestones and the expression of NCAM and PSA-NCAM, key molecules that modulate synaptic plasticity, learning and memory. Our findings reveal subtle deviations in early development while significant reductions in PSA-NCAM were observed throughout adolescence.
Acknowledgement
PSA-NCAM monoclonal Ab: clone 735 was graciously provided by Rita Gerardy-Schahn from Institut für Klinische Biochemie, Medizinische Hochschule Hannover, Germany.
Funding
Research reported in this publication/press release was supported by the National Institute on Drug Abuse of the National Institutes of Health under award number R01DA046723.
Conflicts of Interest
None.
References
- Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, et al. (2017) Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002-2014. JAMA 317(2): 207-209.
- Jones JT, Baldwin A, Shu I (2015) A comparison of meconium screening outcomes as an indicator of the impact of state-level relaxation of marijuana policy. Drug and Alcohol Dependence 156(Supplement C): e104-e105.
- Jarlenski M, Koma JW, Zank J, Bodnar LM, Bogen DL, et al. (2017) Trends in perception of risk of regular marijuana use among US pregnant and nonpregnant reproductive-aged women. Am J Obstet Gynecol 217(6): 705-707.
- Quiroga AS, Alonso JD, Rincón DG, Remmers F, Vega D, et al. (2015) Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB1 receptors on developing cortical neurons. Proceedings of the National Academy of Sciences 112(44): 13693-13698.
- Mereu G, Fà M, Ferraro L, Cagiano R, Antonelli T, et al. (2003) Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proceedings of the National Academy of Sciences 100(8): 4915-4920.
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, Costa BR, et al. (1991) Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci 11(2): 563-583.
- Mackie K (2005) Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol (168): 299-325.
- Berrendero F, Gil LG, Hernandez ML, Romero J, Cebeira M, et al. (1998) Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development 125(16): 3179-3188.
- Mato S, Olmo ED, Pazos A (2003) Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur J Neurosci 17(9): 1747-1754.
- Harkany T, Guzmán M, Roperh IG, Berghuis P, Devi LA, et al. (2007) The emerging functions of endocannabinoid signaling during CNS development. Trends in Pharmacological Sciences 28(2): 83-92.
- Senkov O, Tikhobrazova O, Dityatev A (2012) PSA-NCAM: Synaptic functions mediated by its interactions with proteoglycans and glutamate receptors. Int J Biochem Cell Biol 44(4): 591-595.
- Becker CG, Artola A, Schahn RG, Becker T, Welzl H, et al. (1996) The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res 45(2): 143-152.
- Senkov O, Sun M, Weinhold B, Schahn RG, Schachner M, et al. (2006) Polysialylated neural cell adhesion molecule is involved in induction of long-term potentiation and memory acquisition and consolidation in a fear-conditioning paradigm. J Neurosci 26(42): 10888-109898.
- Pinky PD, Bloemer J, Smith WD, Du Y, Heslin RT, et al. (2023) Prenatal cannabinoid exposure elicits memory deficits associated with reduced PSA-NCAM expression, altered glutamatergic signaling and adaptations in hippocampal synaptic plasticity. Cells 12(21): 2525.
- Holgado FM, Holgado EM, Leret ML, González MI, Reader TA (1993) Distribution of indoleamines and [3H] paroxetine binding in rat brain regions following acute or perinatal delta 9-tetrahydrocannabinol treatments. Neurochem Res 18(11): 1183-1191.
- Qiu J, Singh P, Pan G, Paolis A, Champagne FA, et al. (2020) Defining the relationship between maternal care behavior and sensory development in Wistar rats: Auditory periphery development, eye opening and brain gene expression. PLoS ONE 15(8): e0237933.
- Gao S, Calderon DP (2020) Robust alternative to the righting reflex to assess arousal in rodents. Sci Rep 10(1): 20280.
- Beggiato S, Borelli AC, Tomasini MC, Morgano L, Antonelli T, et al. (2017) Long-lasting alterations of hippocampal GABAergic neurotransmission in adult rats following perinatal Δ9-THC exposure. Neurobiol Learn Mem 139: 135-143.
- Volkow ND, Baler RD, Compton WM, Weiss SR (2014) Adverse health effects of marijuana use. N Engl J Med 370(23): 2219-2227.
- Mariani JJ, Brooks D, Haney M, Levin FR (2011) Quantification and comparison of marijuana smoking practices: Blunts, joints and pipes. Drug and Alcohol Dependence 113(2-3): 249-251.
- Campolongo P, Trezza V, Cassano T, Gaetani S, Morgese MG, et al. (2007) Perinatal exposure to delta-9- tetrahydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addict Biol 12(3-4): 485-495.
- Trezza V, Campolongo P, Cassano T, Macheda T, Dipasquale P, et al. (2008) Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: A longitudinal behavioral study in Wistar rats. Psychopharmacology 198(4): 529-537.
- Fried PA, Watkinson B, Gray R (2003) Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 25(4): 427-436.
- Sengupta P (2013) The laboratory rat: Relating its age with human's. International Journal of Preventive Medicine 4(6): 624-630.
- Fried PA, Watkinson B (2001) Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 23(5): 421-430.
- Kansky J, Allen JP, Diener E (2016) Early adolescent affect predicts later life outcomes. Applied psychology Health and Well Being 8(2): 192-212.
- Krenke IS, Luyckx K, Aro KS (2013) Work and love during emerging adulthood. Emerging Adulthood 2(1): 3-5.
- Breit KR, Rodriguez CG, Lei A, Thomas JD (2020) Combined vapor exposure to THC and alcohol in pregnant rats: Maternal outcomes and pharmacokinetic effects. Neurotoxicol Teratol 82: 106930.
- Moore BF (2024) Prenatal exposure to cannabis: Effects on childhood obesity and cardiometabolic health. Curr Obes Rep 13(1): 154-166.
- Kong KL, Lee JK, Shisler S, Thanos PK, Huestis MA, et al. (2023) Prenatal tobacco and cannabis co-exposure and offspring obesity development from birth to mid-childhood. Pediatr Obes 18(5): e13010.
- Phillips RN, Turk RF, Forney RB (1971) Acute toxicity of Δ9-tetrahydrocannabinol in rats and mice. Proceedings of the Society for Experimental Biology and Medicine 136(1): 260-263.
- Fernandes M, Kluwe S, Coper H (1974) Cannabinoids and hexobarbital induced loss of righting reflexes. Naunyn-Schmiedeberg's Archives of Pharmacology 283(4): 431-435.
- Kimura T, Takaya M, Usami N, Watanabe K, Yamamoto I (2019) ∆9-Tetrahydrocannabinol, a major marijuana component, enhances the anesthetic effect of pentobarbital through the CB1 Forensic Toxicol 37(1): 207-214.
- Wu CS, Jew CP, Lu HC (2011) Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol 6(4): 459-480.
- Muller D, Wang C, Skibo G, Toni N, Cremer H, et al. (1996) PSA-NCAM is required for activity-induced synaptic plasticity. Neuron 17(3): 413-422.
- French ED, Dillon K, Wu X (1997) Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport 8(3): 649-652.
- Hampson RE, Deadwyler SA (2000) Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. The Journal of Neuroscience 20(23): 8932-8942.
© 2024 Vishnu Suppiramaniam and Miranda N Reed, This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






