- Submissions

Full Text
Significances of Bioengineering & Biosciences
3D Printing of Organs and Tissue Constructs
Astha Khanna*
Head - Product Development, Graver Technologies, New Jersey, USA
*Corresponding author:Astha Khanna, Head - Product Development, Graver Technologies, New Jersey, USA
Submission: September 21, 2023 Published: September 28, 2023

ISSN 2637-8078Volume6 Issue3
Abstract
Tissue engineering and regenerative medicine research has utilized Three-Dimensional (3D) bioprinting as a promising technique for fabricating complex functional biological constructs mimicking native tissue for repair and/or replacement of damaged organs or tissues. It has shown how to alleviate the hurdles of conventional tissue engineering methods based on scaffolding and microengineering by precise biomimetic properties and controlled layer-by-layer assembly of biomaterials in a desired 3D pattern. 3D bioprinting involves the top-down approach of building complex tissue with precise geometries using computer graphic generated anatomically accurate 3D models of the tissue. In this comprehensive review, we highlight 3D bioprinting technologies such as ink-jet printing, extrusion printing, stereolithography and laser assisted techniques and applications of 3D bioprinting for construction of tissues such as skin, cardiac, bone and cartilage. We will discuss current challenges with 3D bioprinting technologies and future prospects for advancements for efficient and effective construction of native tissues.
Keywords: 3D printing; Tissue engineering; Inkjet printing; Extrusion-based printing; Cardiac tissue
Introduction
Advancements in tissue engineering has led to engineering and repair of native tissues and organs for regenerative medicine and clinical research. Tissue engineering has been successful in addressing the challenges for regenerating or modeling highly complex and functional tissues. Tissue engineering research involves combination of cells, biomaterials and engineering approaches for fabrication of biological constructs that recapitulate the physiology and function of native tissues and organs [1]. This technique requires formation of an interface cell, scaffolds and growth factors. Scaffolds act as matrices and provide a base for cellular growth and proliferation induced by growth factors. The scaffolds can be fabricated from naturally derived polymers such as gelatin, collagen, hyaluronic acid and alginate, or synthetic polymers such as poly(ϵ-caprolactone) (PCL), Poly (Lactic Acid) (PLA), Poly (Glycolic Acid) (PGA) and Poly (Lactic-Co-Glycolic Acid) (PLGA) [2]. The scaffolds act as 3D templates to support cellular attachment and proliferation followed by cells developing their own ECM that leads to mature cell-laden grafts similar in properties to native tissues [3]. The phenotypes of seeded cells can be regulated by application of different biological or physical stimuli such as growth factors, shear stress, electrical and mechanical cues. However, this approach is time consuming and less efficient as it lacks specific 3D distribution of cells or matrix for mimicking microstructures of biological tissues, hence limiting clinical translation of this technique. It has been studied that the physiologically relevant activities and functions of organs rely on their microarchitectures such as cardiac fibers of myocardium, hepatic lobules of livers and nephron capillary system in kidneys [4].
Additive manufacturing is one of the techniques that has been utilized in tissue engineering and involves principles of material science and biology and involves the top-down approach for generation of complex biomimetic organ and tissue framework in a layer-by-layer fashion. This approach has been successful in generating precise geometries by virtue of controlled matter deposition with the aid of anatomically accurate 3D models of tissue [5]. 3D bioprinting involves building a tissue or organs in a layer-by-layer bottoms up approach and is an extended application of additive manufacturing. The aim is to recapitulate natural cellular architecture by deposition of cells and materials in a manner that can restore structure and function of complex tissues. In the 3D bioprinting approach, cells or biomolecules are directly printed in a specific pattern on a substrate to form a 3D construct [6]. It is an automated process and can provide precise patterning of cells with controlled ECM organization. 3D bioprinting offers unprecedented versatility and potential to deliver cells and biomaterials with precise control over spatial distributions. It is important to consider the modalities associated with the cells and tissues such as biocompatibility of the material used, cell sensitivity to printing methods, growth factor delivery and perfusion etc.
The layer-by-layer structure of bio printed tissues allows for perfusion of gas and nutrients through pores as well as inter and intra cellular talk [7]. One of the essential components in 3D bioprinting is bioink, a composite composed of biomaterials, cells and other components. 3D bioprinting can be divided into direct and indirect fabrications. Indirect bioprinting involves creation of a negative sacrificial mold followed by positive biomaterial cast and selective removal of the mold [8]. Direct bioprinting generates 3D structures in a layer-by-layer manner by depositing multiple cell types and/or biomaterials to achieve 3D constructs. The applications of tissue are not only limited to regeneration of complex damaged tissues in vivo but also includes construction of in vitro models for understanding cellular behaviors and screening of potential therapies using microfluidic organs-ona- chip platforms [9]. However, there are challenges that need to address for successful clinical translation of bio printed tissues and organs such as vascularization, gaseous and nutrient exchange, biocompatibility and biodegradability of the substrate material, shape fidelity and generation of a functional printed tissue. This article aims to provide a comprehensive review of 3D bioprinting process and strategies such as ink-jet printing, stereolithography and laser assisted approaches. This review highlights the overview and application of biomimetic bio printed tissues and organs such as skin, cardiac tissue, blood vessel, bone and cartilage and the challenges associated with the respective bioprinting approaches.
3D Bioprinting Strategies
The process of 3D bioprinting is based on exact layering of biomaterials and has three basis steps: Preparatory phase, Printing phase and Post handling [10]. Preparatory phase involves designing anatomically accurate 3D models using computer graphics such as CAD/CAM and generation of 2D layers of desired thickness to be fed to bioprinter. This step also involves selection of bio-ink or material. The printing phase involves printing of tissues by additive manufacturing. Post-processing involves maturation of fabricated construct and its functional and structural characterization. Bioprinting has been achieved as scaffold based or scaffold free. Scaffold based bioprinting approach involves a biomaterial matrix as a substrate for cellular deposition. The matrix could be a hydrogel, nanofibers or another 3D base that could be used for patterning of bioink. It is important for the 3D matrix to resemble the native ECM microenvironment for cellular attachment, growth and proliferation. In comparison, scaffold free bioprinting involves direct deposition of cellular or tissue aggregates as spheroids that are deposited on print molds via extrusion process [11]. Cells secrete their own ECM that results in a mature tissue that is followed by removal of the mold.
Self-organization of cells increases ECM production and preserves tissue function. Kim et al. [12] studied advances in technologies and materials for engineering 3D cell-printed constructs for therapeutic and drug testing applications [12]. The authors discussed fabrication of complex structures, tissue-specific microenvironments and functional vasculatures for generation of engineered tissues/organs. This review is helpful for understanding characteristics for functional tissues/organs for replacement of damaged tissues and development of in vitro disease modeling and testing platforms for drug development. Different additive manufacturing approaches have been studied for selective cell and biomaterial patterning for generation of viable tissue constructs, for example, inkjet-based 3D bioprinting, extrusion based 3D bioprinting, laser assisted 3D bioprinting, and stereolithographic based 3D bioprinting [13]. Each of the techniques are discussed in the section below.
Inkjet-based 3D bioprinting
Inkjet bioprinting is based on the use of “bio-ink” that refers to a low viscosity suspension biomaterial with active cellular population deposited over a substrate usually a hydrogel, culture dish or polymer. Printing occurs in a digitally controlled pattern as this is a non-contact printing approach. There are two methods to perform Ink-jet printing, a continuous ink-jet printing and drop-ondemand approach. Continuous ink-jet printing involves generation of a continuous jet of droplets by application of pressure to force the bio-ink out of the nozzle followed by application of electric field to deflect the bioink jet onto the substrate [14]. The excess droplets are collected in a gutter as they can be re-used. The dropon- demand method involves production of droplets on demand using a pressure pulse as compared to continuous inkjet printing. One advantage of drop-on-demand printing is its pulsed nature compared to continuous bioprinting where the ink not deflected onto the surface is recirculated through the printer posing a risk of contamination. The drop-on-demand printing approach is categorized into piezoelectric and thermal inkjet printing. Piezoelectric printing uses a piezoelectric transducer in the microfluidic chamber and pulsed voltage applied creates transient pressure for droplet actuation [15].
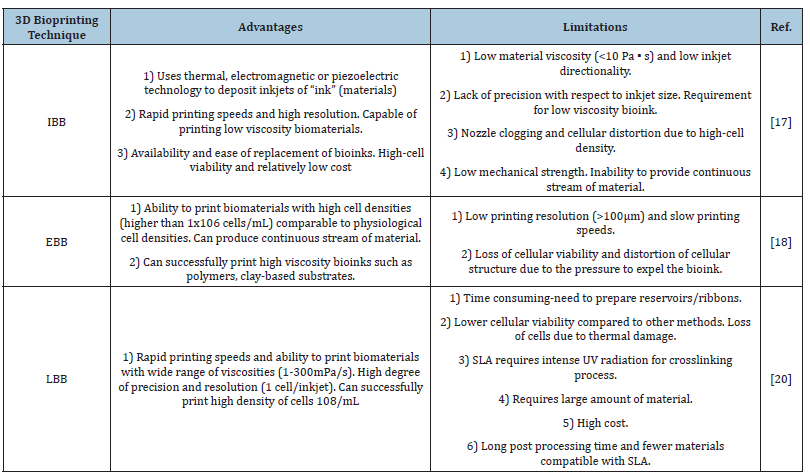
Thermal drop-on-demand printing uses pulsed electric current to a heating element that vaporizes ink droplets in the microfluidic chamber and ink droplet gets pushed by nozzle orifice onto the substrate due to vapor bubble pressure. Cells are exposed to high temperature for a few microseconds and therefore they remain viable as they rise a little above ambient temperature [16]. There are certain attributes that can impact the printing process. The rheological properties of the ink can impact the process with a typical viscosity specification of 30mPa/s that has been studied to be effective. Droplet size is a factor affected by other attributes such as nozzle size, distance between substrate and nozzle, temperature gradient in thermal drop-on-demand printing and transducer properties and frequency of current applied in case of piezoelectric printing. This approach has been successful in mammalian cell printing and DNA/protein patterning. Ink-jet printing has the advantages of relatively inexpensive and non-contact nature that mitigates the risk of contamination. Xu and collaborators recently utilized a drop-on-demand printing approach to develop vascular like alginate tubes by 3D inkjet bioprinting device platform. An advantage of ink-jet printing is the potential of the approach to form complex multicellular patterns and constructs by concurrent printing of multiple cellular types and biomaterials using varied printheads in a single operation [17]. Table 1 includes the advantages and limitations of bioprinting techniques.
Table 1:Advantages and limitations of bioprinting techniques [16].
Abbreviations: IBB: Inkjet Based Bioprinting; EBB: Extrusion Based Bioprinting; LBB: Laser Based Bioprinting
Extrusion based bioprinting
Extrusion based bioprinting can be operated using direct ink bioprinting or pressure assisted bioprinting. Direct ink bioprinting involves a material extrusion process where continuous extrusion of the material from the nozzle occurs generating 3D architecture. The materials used in this approach should specific rheological properties for aid in printing and should be shear thinning to enable extrusion out of the printing nozzle. To induce flow, application of shear stress above the yield stress of the resin is needed as the resin recovers its rigidity on the substrate after the shear stress is released. To achieve desired rheological properties, the polymer resins are blended with fillers such as silica particles that induce shear thinning flow behavior [18]. These properties allow for shape retention of the printed structure. Alternative processes such as UV curing of the printed layer, thermal cure of extrusion into a support bath can aid solidifying as the latter holds the structure in place until conversion of deposited ink into a solid. This process is also called ‘Freeform Reversible Embedding’ (FRE) or embedded 3D bioprinting. Traditional scaffolding processes such as solvent casting, electrospinning and salt leaching have the limitation to control pore architecture provided by CAD/CAM processes.
Traditionally, pressure assisted deposition was used to address this concern. Polymers used primarily in this process are Polycaprolactone (PCL), Polylactide (PLA) and their blends or composites with ceramics. With the advancement of organ bioprinting, cell-encapsulated hydrogels have been utilized. Yin and collaborators employed pressure-assisted multi-syringe deposition platform to encapsulate rodent hepatocytes in gelatin hydrogels in conjunction with alginate, chitosan and fibrinogen for generation of a functional liver construct [19]. While the construct exhibited cellular viability and function, enzymatic degradation affected construct stability even though the approach was successful in simultaneous deposition of cells and biomaterials. Gelatinbased hydrogels have been utilized for generation of biomimetic 3D hepatocyte and adipose-derived stem cells constructs. Incorporation of cells in Matrigel® for applications of bone grafts have been utilized to generate multi-cellular biomimetic constructs.
Laser assisted 3D bioprinting or laser induced forward transfer
Deposition of cells on substrate is carried out using a pulsed laser beam that makes this a non-contact 3D printing process. There are three elements to Laser Assisted 3D Bioprinting process: a pulsed laser beam source, a ribbon coated with bioink and a substrate onto which the bioink is deposited. The energy source is usually UV lasers or near wavelength lasers with nanoscale pulse wavelength. The bioink coated is on a target plate made of a material that allows transmission of laser through it such as quartz. The substrate is coated with a natural polymer or nutrient medium to facilitate the cellular deposition and growth. Application of laser pulse causes volatile cell laden ink propelled onto the substrate. This led to the innovation of ‘absorbing film-assisted laser-induced forward transfer (AFA-LIFE)’ or Biological Laser Processing (BioLP)’ and Matrix-Assisted Pulsed Laser Evaporation Direct Writing (MAPLEDW) [20].
Laser Induced forward transfer was developed using highenergy laser pulse for direct writing of metal features on an optical substrate. This approach was extended for printing of biomolecules in the form of AFA-LIFE and BioLP. The interface of ribbon and bioink has a sacrificial layer of laser absorbing layer of any metal/ oxide such as Ti, TiO2 to protect cells from laser exposure. High energy laser beam causes thermal expansion of the sacrificial layer that results in propulsion of bio-ink onto the substrate with minimal cell damage. BioLP process uses a low-powered pulsed-laser and a hydrogel sacrificial layer such as Matrigel®. The hydrogel binds the bio-ink and the ribbon [21]. Cells can be printed as encapsulated in ECM-like biomaterial or directly printed onto/in ECM layer. Factors that can affect cellular viability during printing are ECM thickness onto which cells are deposited, laser-pulse energy, bioink viscosity etc. High laser pulse energy has been studied to cause cell fatality, and increased thickness of sacrificial layer and bioink viscosity results in cellular viability [22].
Stereolithographic based 3D bioprinting
The Stereolithographic bioprinting method employs addition of materials by projecting light on a photo-sensitive heat-curable bioink in a plane-by-plane fashion. The approach uses light as a cross-linking agent and thus the ink is required to have some photo-curable moieties such as acrylate derivatives of Polyethylene Glycol (PEG) such as PEG Dimethacrylate (PEGDMA) and PEG Diacrylate (PEGDA) utilized in photo-polymerization of scaffolds in tissue engineering [23]. Stereolithographic printing has two broad categories, a) Single photon method and multi-photon method. The single photon method has sub-components -1) Visible radiation systems, 2) traditional stereolithography, 3) IR stereolithography and 4) Stereo-thermal lithography. Traditional single-photon Stereolithography Apparatus (SLA) can facilitate crosslinking of UV sensitive fluid oligomers into sol-gel polymeric networks utilizing UV photons [24]. Polymerization by a photosensitive resin has been studied in 3 steps: initiation, elongation and termination.
Stereolithographic method has been utilized for fabrication of biocompatible and biodegradable scaffolds.
Advincula and collaborators studied SLA polymerization of UV curable polymer-ceramic composites used for cellular seeding in a minipig model bone regeneration model [24]. Another group studied fabrication of vinyl ester resin bone regeneration scaffold by using vinyl ester resin in a rabbit model for bone growth and regeneration [25]. In cartilage tissue engineering, a bioabsorbable scaffold based on hyaluronic acid derivative, Hyaff1a has been fabricated using UV lasers based stereolithography and clinical imaging. Inui and collaborators created a 2D cardiac tissue as cell sheets using primary neonatal cardiomyocytes through stereolithographic approach [26]. Another group studied a trileaflet heart valve fabricated using stereolithography bioprinting of polyhydroxy octanoate and elastomers of poly-4-hydroxybutyrate [27]. Direct laser writing was utilized to fabricate polyethylene glycol hydrogels with fibroblasts secreting Vascular Endothelial Growth Factor (VEGF) like molecules. Two photon laser scanning photolithography was utilized to create 3D liver tissue construct via laser polymerization of photosensitive polymers followed by functionalization with collagen seeded with rat hepatocytes.
Applications of 3D Bioprinting for Organ Regeneration
Skin tissue 3D bioprinting
Human skin is a complex structure with major components of the tissue as epidermis and dermis and subcutaneous part forming the third region. Skin serves the functions of protecting from UV rays, prevents drying and acts as a barrier to prevent entry of toxins and pathogens. Skin is also the first line of immune system defense. The upper epidermis layer is primarily composed of keratinocytes organized as keratinized stratified squamous epithelium. The growth of epidermis occurs from inwards to outwards with the basal layer having proliferating keratinocytes and mature cells on the surface. The basal layer or basement membrane acts as the separation between epidermis and dermis. The proliferative cells undergo differentiation with the newer undifferentiated cells at the bottom and terminally differentiated cells in stratum corneum on the outside. Melanocytes secrete melanin that protects the skin from UV rays and provide the skin pigmentation and color. Other cells in the epidermis are nerve endings and glandular ducts and immune cells such as Langerhans cells and T cells. The second layer of the skin called dermis has two layers: the upper papillary and the lower rectal dermis. The upper papillary dermis is composed of loose, areolar connective tissue with dermal papillae and high ratio of collagen III and the lower reticular dermis is made up of dense connective tissue with high amount of collagen I. This difference in the collagen in the extracellular matrix of dermis imparts elasticity and mechanical strength to the skin.
Bioprinting the skin is of great significance because of its robustness and vitality. One of the earliest advancements in this area was the creation of artificial skin grafting that could be used as a bandage for wound and burn healing. Bioprinting the skin construct has been studied by some research groups and has advantages over conventional approaches in that the process is automated and standardized for the specific clinical application that ensures precision in cellular deposition [28]. Traditional scaffold-based culture methods are time consuming with long production times to obtain constructs with large surface area. Bioprinting can be performed either in situ, at the site of injury or in vitro, where the construct is allowed to mature in a bioreactor before transplantation. In situ bioprinting provides higher precision in cellular deposition on the wound and can obviate the need for use of expensive biomaterials in less time compared to in vitro differentiation.
Skin tissue 3D bioprinting process
The bioprinting process for skin tissue consists of four steps. The first step is known as pre-processing and involves cell and biomaterial selection. The next step is the printing process followed by post processing that involves cell proliferation and maturation of bio printed skin construct. The last step is characterization of printed tissue and evaluation of its function. Cellular population for printing is obtained from skin biopsy and is expanded via cell culture techniques. The cells could be either primary cells from a healthy donor or can be stem cells if donor has injured skin. Stem cells are derived from either adipose cells or mesenchymal cells or prenatal cells. Clinical images obtained from MRI or PET could be used as an input to design anatomically accurate models of the functional tissue using CAD/CAM graphic interface [29].
The STL model can be sliced into layers followed by deposition of the bio-ink. The thickness of the slices usually lies in the 100-500 micrometer range for inkjet and extrusion based bioprinters and a resolution of 20-100 micrometers for laser assisted bioprinting. Printing resolutions below 100 micrometers provide precise patterning of cell-laden constructs. A pre-requisite to precision bioprinting is high quality image acquisition from clinical imaging as quality of fabricated construct depends on the accuracy of the anatomical model [29]. However, although in vivo cell distribution can be obtained by clinical imaging, it is challenging to use image processing tools to get anatomically accurate skin geometry. Hence, maturation of the printed construct is needed especially in in vitro bioprinting where the printed tissue matures in a bioreactor in comparison to in situ printing where maturation occurs at the site of injury.
Bioinks for bioprinting skin tissue
Bioink used for the bioprinting purpose should possess the biomechanical properties needed for deposition of ink in the patterns per stereolithographic approach. This is essential as bioink facilitates the cellular-ECM interactions and impacts cellular growth and proliferation. Not only the bioink should be biocompatible, but it must also support the structure and function of the printed tissue and promote cellular differentiation. The choice of biomaterials used for bioinks can range from natural polymers as gelatin, collagen, hyaluronic acid, aliginate to synthetic polymers such as Polyethylene Glycol (PEG), poly(lactic-co-glycolic acid) (PLGA), Polycaprolactone (PCL) or a hybrid of different polymers. Sacrificial support materials such as Pluronic F-127 could be used to support and keep the cells together. Factors that are considered to select bioink for a specific application are rheological properties of the ink, gelation kinetics, shape fidelity and printing resolution of the ink. Zhang and collaborators used bio-ink suspension composed of Amniotic Fluid Derived Stem Cells (AFSCs) and bone marrow derived stem cells suspended in thrombin crosslinked fibrin-collagen printed at the injury site [30]. Human keratinocytes and fibroblasts were printed on athymic nude mice using ink-jet printing that resulted in re-epithelialization in 7 weeks.
3D bioprinting liver tissue
Liver has a functional unit called hepatic globule, a hexagonal structure that facilitates complex exocrine and endocrine functions such as metabolism and detoxification. The parenchymal hepatocytes have an endothermic origin and form a significant portion of liver. Cells that compose the liver are fibroblasts, sinusoidal endothelial cells and biliary epithelial cells in addition to mesoderm derived cells such as Hepatic Stellate Cells (HSC), Kupffer cells and stromal cells. The non-parenchymal cells are involved in important functions like synthesis of growth factors and ECM proteins facilitated by HSCs that regulate homeostasis and cellular signaling. Collagen and glycosaminoglycan regulate cell signaling and mechanical integrity of hepatocytes. Traditional 2D culture methods have been employed for fabrication of biomimetic liver tissues; however, these methods have the limitations of creating the microenvironment for cell-ECM interactions thus limiting cellular survival rate. Some studies have pointed out the role of intercellular adhesion for construction of volumetric liver tissues. 3D bioprinting has been studied to fabricate liver-like microstructures.
Liver hepatocytes and hepatoma cells have been printed using hydrogels such as MeHA, PEG, alginate and gelatin. One of the pioneers in bioprinting, Organovo TM successfully fabricated vascularized liver constructs with high cellular viability through bioprinting of high-density hepatocytes, endothelial cells and hepatic stellate cells forming an architecture resembling native hepatic lobules [31]. In addition, liver spheroids have been utilized in bioprinting as a replacement for single hepatocytes. Liver spheroids have been studied to recapitulate the volumetric cell-cell interactions and protect the cells from shear stress via printing process. Ma and collaborators studied bio printed liver spheroid embedded in Gel MA hydrogel for long-term viability and functionality as evidenced by secretion of hepatic biomarkers such as albumin, transferrin, alpha 1 antitrypsin [32]. Liver spheroids with microfluidic bioreactors was studied as a viable platform to screen hepatotoxic drugs for dose and time dependent responses of organoids for secretion of biomarkers.
3D bioprinting of cardiac tissue
Cardiovascular diseases account for leading cause of mortality worldwide with an estimate of total incidents of myocardial infarction per year of about 8 million [33]. One of the implications of cardiovascular diseases is the loss of irreplaceable cardiomyocytes as these cells have no repair or regeneration mechanism. Instead, the loss of cardiomyocytes leads to formation of a non-functional scar tissue with high risk of acute cardiomyopathy. These conditions are currently managed by bypass grafting of coronary arteries, cellular therapy, left ventricular assist device and heart transplantation. There are risks associated with these treatments in addition to lack of suitable donors for transplant and immune rejections. Tissue engineering aims to provide solutions to these obstacles in treatment for cardiovascular diseases. Traditional approaches to cardiovascular tissue engineering involve the growth and proliferation of functional biomaterial scaffolds to support differentiation of stem cells. Decellularized scaffolds have been studied along with synthetic and natural hydrogels for biocompatibility and resemblance to the native tissue cell matrix. Autologous and allogenic stem cells have been studied in cardiac tissue engineering for lesser risk of immune rejections.
Recapitulating the complexity of a functional cardiac tissue is a challenge and requires integration of multiple cellular types such as cardiomyocytes, fibroblasts and endothelial cells. Other challenges include attaining the auto-rhythmic nature of myocardium. 3D bioprinting has gained interest in overcoming these challenges and limitations in building a functional tissue construct and restoration of characteristics of native cardiac tissues. Extensive research has been performed in fabrication of functional myocardium and heart valves.
Cardiac bioprinting involves pre-processing, printing process and post processing steps. The first step is the creation of a 3D model for bioprinting a functional cardiac construct utilizing CAD/ CAM modeling interfaces and clinical imaging techniques such as MRI and CT scans [34]. High fidelity models can be created using imaging methods such as nuclear imaging PET and volumetric 3D echocardiography. Generation of patient specific 3D cardiovascular model has been achieved with image segmentation processes. Firstly, identification of anatomic geometry of the target tissue is performed using clinical imaging dataset exported into a digital imaging. The target cardiac tissue is then segmented into 2D projections in different anatomical planes such as axial, sagitta and coronal. Next, 2D images are stacked in the form of segmentation masks for printing that are then rendered into stereolithographic files to assess patient specificity. The files are then exported to bioprinter for tissue construct printing. The tissue construct undergoes maturation in a bioreactor to customize the construct for parameters such as contraction etc. These are characterized for mechanical and electrical stimulation to assess if long-term sustenance of contractions and relaxations is possible while maintaining tissue morphology. One of the challenges with cardiac bioprinting is maintaining perfusion of heart tissue in postprocessing of printed cardiac constructs.
Bioinks used for cardiac bioprinting should meet criteria such as spatial control of hydrogel deposition by formation of stable elements via crosslinking mechanisms [35]. Maintenance of cellular viability is an important criterion for cardiac bioprinting. Natural and synthetic polymers have been used such as collagen, gelatin, hyaluronic acid etc. for bioink formation. Cardiac bioprinting can be performed with or without scaffolding. Scaffold utilized in cardiac bioprinting can be pre-printed and seeded with cells to be printed simultaneously while scaffold-free process involves direct printing of biomolecules and cells on the substrate. Summarizes biomaterials that have bene utilized for cardiac tissue bioprinting with the research outcomes of the bioprinting process.
3D bioprinting of cartilage tissue
Articular cartilage has a complex structure formed of biomolecules such as collagen, proteoglycans and non-collagenase proteins. The cartilage is made of chondrocytes embedded in an extracellular matrix. The tissue is capable of withstanding intensive and repetitive physical stress but can be degraded by mechanical, chemical and microbiological agents resulting in an injury. The avascular nature of the cartilage tissue and lack of a lymphatic and nervous system, the injury due to trauma or excessive stress cannot be regenerated and can result in several degenerative diseases such as osteoarthritis that depletes the patient’s quality of life. The current treatments include microfracture, osteochondral grafts, autologous implantation and therapies such as MACI (autologous chondrocytes cultured on porcine collagen) [36], However, these treatments provide short-term clinical solution and an inferior cartilage function. Traditional tissue engineering aims to homogenously distribute the biological factors across the tissue; however, the cartilage is composed of three zones that differ in gradients of collagen, proteoglycans and arrangement of proteoglycans. Thus, there has been significant attention to the development of alternate therapies. 3D bioprinting has achieved controlled and successful generation of spatial patters and different grades of cartilage tissue to imitate cartilage anatomy, although the different regions of articular cartilage consist of variations in cellular densities, morphologies, mucopolysaccharides composition and mechanical characteristics.
3D bioprinting of cartilage involves six steps that include. a) Imaging, b) designing of replacement tissue, c) Material preparation, d) cellular preparation, e) Bioprinting, f) Implantation [37]. The primary step in cartilage tissue bioprinting is generation of patient specific medical image through imaging techniques such as MRI/CT scans and digitized by CAD/CAM. The biomaterials utilized in 3D bioprinting must have some properties such as printability, wetting and swelling characteristics, degradation kinetics and stability of structures. The material preparation step involves encapsulation of chondrocytes and stem cells into alginate hydrogels that can retain cellular viability and metabolic function. The cellular components should support ECM synthesis, vascularization and generation of nervous system and should be robust enough to survive the printing process and intact cellular function. The mechanisms utilized for cartilage bioprinting are Inkjet based, extrusion based and stereolithography. Selection of appropriate bioink on the basis of composition and mechanical properties is critical. The main bioinks utilized for cartilage tissue bioprinting are natural polymers such as collagen, fibrin etc. and synthetic polymers such as Polyethylene Glycol (PEG). Martinez and collaborators utilized hyaluronic acid with Poly-Lactic Acid (PLA) to formulate a novel bioink for cartilage tissue 3D bioprinting [38]. Self-gelling property of silk fibroin with gelatin has been utilized as cross-link free bioink for cartilage tissue bioprinting.
Challenges and Future Prospects of 3D Bioprinting Techniques
3D bioprinting offers great potential for tissue and organ regeneration as it allows for fabrication of physiologically relevant tissues with relevant and consistent functional outcomes in patients. 3D bioprinting could be the alternative to organ transplantation for its potential to successfully integrate and construct a tissue with high scalability, stability and semblance to native structures [39]. This approach allows for viable and high throughout tissue printing with better spatial control and precise patterning of cells in comparison with traditional tissue culture methods. One is the challenge in 3D bioprinting is manufacturing a mechanically stable 3D construct. Porosity and structure designed by 3D bioprinting should have a high elastic modulus to support the natural cellular growth during implantation [40]. In the absence of proper structural support, the newly formed tissues can fail. Panja et.al. studied 3D bioprinting and decellularization technique for generation of patient specific organs and tissues. Biomaterial and bioink-based stereolithography have been utilized most extensively for 3D orienting tissues and organs [41].
Vascularization of the bio printed construct is another important criterion that ensures transport of growth factors, oxygen, nutrients and waste removal [42]. In addition to obtaining bio printed constructs for clinical translation, it is important for integration of a functional vasculature in the grafts for long term cellular survival. Different tissues require different cellular densities, cellular types and spatiotemporal distribution inside the 3D printed constructs [42]. Further, in application involving stem cells, different matrix properties may modulate differentiation and trans-differentiation of cells into specific lineages. Xia et al. [43] discusses the state-of-the-art advancements in use of biomaterials in 3D bioprinting for tissue fabrication. The authors highlighted 3D tissue and organ printing with a specific focus on 3D printing of vascularized tissue and organ constructs [43]. Future developments in the 3D bioprinting field can lead to rapid developments in bioprinters to perform the 3D printing process with high resolution with adequate mechanical strength and cellular viability [44]. Development of bioinks with optimized bio printability and bio functional properties are important for success in clinical translation of 3D printed tissues and organs. Maintenance of cellular viability in the bioink formulation followed by printing in precise geometries needs standardization of the printing methods and for effective and efficient fabrication of the printed product [45- 49]. Finally, there is also need for better assays to analyze cellular functionality in 3D printed constructs for development of effective functional constructs. All of these developments will lead to success in clinical translation and rapid drug development thereby reducing significant costs associated with drug screenings.
Conclusion
3D bioprinting has shown great promise in fabrication of physiologically relevant tissue and organs with spatial, mechanochemical and temporal characteristics. 3D multi-cellular organ typical constructs can mimic the in vivo physiological environment and can be utilized for in vivo cellular studies, high throughout drug screening, disease modeling and regenerative medicine. While 3D printing has been successful in advancements in fabrication of engineered tissues and organs, there are challenges that need be addressed. Firstly, it is challenging to recapitulate the complexity of extracellular matrix and intrinsic cellular morphologies and functions. 3D bioprinting has been utilized to generate vascular networks and recapitulate cellular configurations of complex tissue architecture, however, generation of dense capillaries for transport of oxygen and nutrients is for each cell on a microscopic scale is challenging. This restricts the size of the fabricated constructs to ≤0.5 mm thickness. While 3D bioprinting is a significant advancement to earlier cell culture models for disease modeling, drug screening and tissue regeneration, there are certain challenges that need to be addressed for successful clinical translation of engineered tissues and biomaterials.
References
- Mandrycky C, Phong K, Zheng Y (2017) Tissue engineering toward organ-specific regeneration and disease modeling. MRS Commun 7(3): 332-347.
- Chen M, Jiang R, Deng N, Zhao X, Li X, et al. (2022) Natural polymer-based scaffolds for soft tissue repair. Front Bioeng Biotechnol 10: 954699.
- Thompson CL, Fu S, Knight MM, Thorpe SD (2020) Mechanical stimulation: A crucial element of organ-on-chip models. Front Bioeng Biotechnol 8: 602646.
- Heinonen I, Kalliokoski KK, Hannukainen JC, Duncker DK, Nuutila P, et al. (2014) Organ-specific physiological responses to acute physical exercise and long-term training in humans. Physiology 29(6): 421-436.
- Agarwal S, Saha S, Balla VK, Pal A, Barui A, et al. (2020) Current developments in 3D bioprinting for tissue and organ regeneration-a review. Frontiers in Mechanical Engineering 6.
- Rafelski SM, Marshall WF (2008) Building the cell: Design principles of cellular architecture. Nature Reviews Molecular Cell Biology 9(8): 593-602.
- Dey M, Ozbolat IT (2020) 3D bioprinting of cells, tissues and organs. Scientific Reports 10(1): 14023.
- Zhang YS, Yue K, Aleman J, Moghaddam KM, Bakht SM, et al. (2017) 3D bioprinting for tissue and organ fabrication. Ann Biomed Eng 45(1): 148-163.
- Gaharwar AK, Singh I, Khademhosseini A (2020) Engineered biomaterials for in situ tissue regeneration. Nature Reviews Materials 5(9): 686-705.
- Vanaei S, Parizi MS, Vanaei S, Salemizadehparizi F, Vanaei HR (2021) An overview on materials and techniques in 3D bioprinting toward biomedical application. Engineered Regeneration 2: 1-18.
- Yu Y, Moncal KK, Li J, Peng W, Rivero I, et al. (2016) Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands” as a new bioink. Scientific Reports 6(1): 28714.
- Kim J, Kong JS, Han W, Kim BS, Cho DW (2020) 3D cell printing of tissue/organ-mimicking constructs for therapeutic and drug testing applications. Int J Mol Sci 21(20): 7757.
- Zhang W, Liu Y, Zhang H (2021) Extracellular matrix: An important regulator of cell functions and skeletal muscle development. Cell & Bioscience 11(1): 65.
- Cui X, Boland T, D'Lima DD, Lotz MK (2012) Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv Formul 6(2): 149-155.
- Bernasconi R, Brovelli S, Viviani P, Soldo M, Giusti D, et al. (2022) Piezoelectric drop-on-demand inkjet printing of high-viscosity inks. Advanced Engineering Materials 24(1): 2100733.
- Plog J, Jiang Y, Pan Y, Yarin AL (2020) Electrostatic charging and deflection of droplets for drop-on-demand 3D printing within confinements. Additive Manufacturing 36: 101400.
- Khanna A, Zamani M, Huang NF (2021) Extracellular matrix-based biomaterials for cardiovascular tissue engineering. J Cardiovasc Dev Dis 8(11): 137.
- Varchanis S, Haward SJ, Hopkins CC, Syrakos A, Shen AQ, et al. (2020) Transition between solid and liquid state of yield-stress fluids under purely extensional deformations. Proceedings of the National Academy of Sciences 117(23): 12611-12617.
- Yin X, Ren J, Lan W, Chen Y, Ouyang M, et al. (2022) Microfluidics-assisted optimization of highly adhesive haemostatic hydrogel coating for arterial puncture. Bioactive Materials 12: 133-142.
- Malinauskas M, Žukauskas A, Hasegawa S, Hayasaki Y, Mizeikis V, et al. (2016) Ultrafast laser processing of materials: From science to industry. Light Sci Appl 5(8): e16133-e16133.
- Ringeisen BR, Othon CM, Wu X, Krizman DB, Darfler MM, et al. (2010) Biological Laser Printing (BioLP) for high resolution cell deposition. In: Ringeisen BR, Spargo BJ, Wu PK (Eds.), Cell and organ printing. Dordrecht, Springer, Netherlands, pp. 81-93.
- Keriquel V, Oliveira H, Rémy M, Ziane S, Delmond S, et al. (2017) In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Scientific Reports 7(1): 1778.
- Sharifi S, Sharifi H, Akbari A, Chodosh J (2021) Systematic optimization of visible light-induced crosslinking conditions of gelatin methacryloyl (GelMA). Scientific Reports 11(1): 23276.
- Thomas S, Senellart P (2021) The race for the ideal single-photon source is on. Nature Nanotechnology 16(4): 367-368.
- Jang JW, Min KE, Kim C, Shin J, Lee J, et al. (2023) Review: Scaffold characteristics, fabrication methods, and biomaterials for the bone tissue engineering. International Journal of Precision Engineering and Manufacturing 24(3): 511-529.
- Inui A, Sekine H, Sano K, Dobashi I, Yoshida A, et al. (2019) Generation of a large-scale vascular bed for the in vitro creation of three-dimensional cardiac tissue. Regenerative Therapy 11: 316-323.
- Hasan A, Soliman S, Hajj FE, Tseng YT, Yalcin HC, et al. (2018) Fabrication and in vitro characterization of a tissue engineered PCL-PLLA heart valve. Scientific Reports 8(1): 8187.
- Olejnik A, Semba JA, Kulpa A, Pazdrowska AD, Rybka JD, et al. (2022) 3D bioprinting in skin related research: Recent achievements and application perspectives. ACS Synthetic Biology 11(1): 26-38.
- Herrmann KA, Kohan AA, Gaeta MC, Rubbert C, Conejero JL, et al. (2013) PET/MRI: Applications in clinical imaging. Current Radiology Reports 1(3): 161-176.
- Zhang Y, Yan J, Liu Y, Chen Z, Li X, et al. (2021) Human amniotic fluid stem cell-derived exosomes as a novel cell-free therapy for cutaneous regeneration. Frontiers in Cell and Developmental Biology 9: 685873.
- Zhang YS, Haghiashtiani G, Hübscher T, Kelly DJ, Lee JM, et al. (2021) 3D extrusion bioprinting. Nature Reviews Methods Primers 1(1): 75.
- Ma LD, Wang YT, Wang JR, Wu JL, Meng XS, et al. (2018) Design and fabrication of a liver-on-a-chip platform for convenient, highly efficient, and safe in situ perfusion culture of 3D hepatic spheroids. Lab Chip 18(17): 2547-2562.
- Tsao CW, Aday W, Almarzooq IZ, Alonso A, Beaton AZ, et al. (2022) Heart disease and stroke statistics update: A report from the american heart association. Circulation 145(8): e153-e639.
- Wang Z, Wang L, Li T, Liu S, Guo B, et al. (2021) 3D bioprinting in cardiac tissue engineering. Theranostics 11(16): 7948-7969.
- Tarassoli SP, Jessop ZM, Jovic T, Hawkins K, Whitaker IS (2021) Candidate bioinks for extrusion 3D bioprinting-a systematic review of the literature. Frontiers in Bioengineering and Biotechnology 9: 616753.
- Vyas C, Mishbak H, Cooper G, Peach C, Pereira RF, et al. (2020) Biological perspectives and current biofabrication strategies in osteochondral tissue engineering. Biomanufacturing Reviews 5(1): 2.
- Zhang J, Wehrle E, Rubert M, Müller R (2021) 3D bioprinting of human tissues: Biofabrication, bioinks, and bioreactors. Int J Mol Sci 22(8): 3971.
- Cabral LR, Teixeira LN, Gimenez RP, Demasi AP, Brito RB, et al. (2020) Effect of hyaluronic acid and poly-l-lactic acid dermal fillers on collagen synthesis: An in vitro and in vivo Study. Clin Cosmet Investig Dermatol 13: 701-710.
- Khanna A (2017) Fabrication of human serum albumin film for enhanced hemocompatibility and mitigation of neointimal hyperplasia under physiologically relevant flow shear conditions. Clemson University, USA.
- Lu X, Khanna A, Luzinov I, Nagatomi J, Harman M (2018) Surface modification of polypropylene surgical meshes for improving adhesion with poloxamine hydrogel adhesive. J Biomed Mater Res Part B Appl Biomater 107: 1047-1055.
- Panja N, Maji S, Choudhuri S, Ali KA, Hossain CM (2022) Bioprinting of Human Hollow Organs. AAPS PharmSciTech 23(5): 139.
- Khanna A, Oropeza BP, Huang NF (2022) Engineering spatiotemporal control in vascularized tissues. Bioengineering 9(10): 555.
- Xia Z, Jin S, Ye K (2018) Tissue and organ 3D bioprinting. SLAS Technology 23(4): 301-314.
- Khanna A, Ayan B, Undieh AA, Yang YP, Huang NF (2022) Advances in three-dimensional bioprinted stem cell-based tissue engineering for cardiovascular regeneration. J Mol Cell Cardiol 169: 13-27.
- Khanna A (2015) Fabrication of human serum albumin film for enhanced hemocompatibility and vascular compatibility. Transactions of the Annual Meeting of Society for Biomaterials.
- Khanna A (2017) Fabrication of human Serum Albumin film on expanded polytetrafluoroethylene for enhanced hemocompatibility and adhesion strength. Transactions of the Annual Meeting of Society for Biomaterials.
- Khanna A (2023) Extracellular matrix bioactive molecules and cell behavior modeling. In: Maia RF, Oliveira JM, Reis RL. Cham Handbook of the Extracellular Matrix: Biologically Derived Materials. Springer International Publishing, pp. 1-18.
- Khanna A, Oropeza BP, Huang NF (2023) Growth Factors Regulation in Angiogenesis. Encyclopedia.
- Khanna A Oropeza BP, Huang NF (2023) Cardiovascular human organ-on-a-chip platform for disease modeling, drug development, and personalized therapy. J Biomed Mater Res 1-12.
© 2023 Astha Khanna, This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






