- Submissions

Full Text
Significances of Bioengineering & Biosciences
Advances in Biomedical Imaging Techniques: A Comprehensive Reviews
Mubashar Ilyas1,2, Saniyah Amin2,3, Maida4, Afsheen Saba1,2, Talha Baig1, Maroof Ahmad Khan*1 and Sabahat Imran5
1Key Laboratory of Cluster Science of Ministry of Education, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, China
2Department of Chemistry, University of Agriculture, Pakistan
3University of Education, Lahore, Pakistan
4Bahauddin Zakariya University, Multan, Pakistan
5Government College University Faisalabad, Pakistan
*Corresponding author:Maroof Ahmad Khan, Key Laboratory of Cluster Science of Ministry of Education, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, China
Submission: July 03, 2023 Published: July 26, 2023

ISSN 2637-8078Volume6 Issue2
Abstract
Healthcare and research have significantly improved as a result of the adoption of biomedical imaging techniques. These technologies enable non-invasive visualization and characterization of biological structures and functions. This review will examine the most current developments in biomedical imaging in this extensive study, with a focus on diverse modalities and their applications. To start, we’ll give a general introduction to X-ray imaging, including its foundational ideas as well as recent technical developments. Then, this will look at Magnetic Resonance Imaging (MRI), covering its underlying theories, medical uses and recent developments. This will also go through the underlying ideas, diagnostic applications and technical advancements of ultrasound imaging. This will also examine Computed Tomography (CT) imaging, concentrating on its fundamentals, clinical use and most recent developments. Additionally, this review will discuss the fundamentals, uses and current issues related to Positron Emission Tomography (PET) imaging, specifically how it works in conjunction with other modalities.
We will also examine fluorescence and bioluminescence imaging techniques, as well as the advancing technology behind them and their potential applications. This review will also delve further into molecular imaging, highlighting its significance in precision medicine and the most current developments in imaging probes and contrast agents. The review will also go over how crucial image analysis and computational methods like segmentation, registration, and deep learning algorithms are to biomedical imaging. This review will examine the problems that are now plaguing the area and provide recommendations for potential solutions in the future. This review will also examine how biomedical imaging affects healthcare and personalized treatment. The overall objective of this study is to provide a complete evaluation of biomedical imaging developments, highlighting their potential to increase research, diagnosis and therapy across a range of medical specialties.
Keywords:Biomedical Imaging; Positron Emission Tomography (PET) Imaging; X-ray imaging
Introduction
Biomedical imaging techniques have changed the way that healthcare and biomedical research are conducted by enabling non-invasive visualization and characterization of biological structures and processes [1,2]. These imaging modalities, which range from X-rays through MRI, ultrasound, Computed Tomography (CT), Positron Emission Tomography (PET), optical imaging and molecular imaging, have changed how we can study, identify and treat a variety of diseases [3,4]. The breadth and precision of imaging modalities have expanded due to ongoing advancements in imaging technology and procedures, offering priceless insights into the human body and biological processes [5,6]. It is impossible to overstate the value of biomedical imaging in healthcare [7]. These methods, which take exact images of anatomical structures, physiological processes and molecular interactions, are essential for diagnosing illnesses, arranging treatments and monitoring therapy outcomes. Several medical specialties, including radiology, cardiology, neurology, oncology and orthopedics, employ biomedical imaging. It is also a helpful tool for preclinical research, enabling researchers to see and investigate biological processes in real-time animal models, advancing both fundamental and pharmaceutical research [8].
Significant progress has been achieved in improving and enhancing the capabilities of each imaging modality throughout the years [9]. Clinical practice and research outcomes have changed as a result of new imaging technology, enhanced image acquisition and reconstruction methods and the use of multimodal imaging methodology. In this comprehensive research, we intend to examine recent advances in biomedical imaging techniques. This will examine the foundations, clinical applications and technological developments of each modality. This will start with X-ray imaging and look at both its core ideas and the most current developments in the field [10]. The next section of this indepth study will detail how this review plan to analyses current developments in biomedical imaging technology. This review will discuss each modality’s guiding principles, therapeutic uses and technical developments. This review will explore the fundamental ideas, practical applications and emerging developments in each field of imaging, starting with X-rays and moving on through MRI, ultrasound, CT, PET, optical and molecular imaging [11]. This review will also discuss computational methods for imaging of living things and image processing, such as deep learning algorithms, segmentation and registration. These analytical techniques are essential for deriving information from imaging data that may be used for quantitative analysis and automated diagnostic systems [12]..
This review will also go through the challenges and limitations that biomedical imaging faces, including picture quality, acquisition speed, accessibility and the requirement for improved contrast agents. This review will also talk about prospective solutions and openings, such technological improvements, data integration, AI integration and ML integration. Finally, the aim of this study is to provide a comprehensive overview of the most recent developments in biomedical imaging to academics, clinicians and healthcare professionals [13]. By highlighting the possible uses, difficulties and future opportunities, we want to stimulate more study, cooperation and innovation in this quickly developing sector. The continuous progress in biomedical imaging techniques holds great promise for advancing personalized medicine, improving patient outcomes and unravelling human biology’s mysteries [14].
X-Ray Imaging
Principles and mechanisms of X-ray imaging
One of the most extensively utilized and well-established medical imaging modalities is X-ray imaging, sometimes known as radiography [15]. It uses the idea of X-ray attenuation to provide detailed pictures of the human body’s interior architecture. X-rays are a kind of electromagnetic radiation that emits more energy than visible light [16]. A block diagram of the X-ray machine is shown in Figure 1. In X-ray imaging, a controlled X-ray beam is aimed towards the body portion of interest. As X-rays travel through the body, they are attenuated or absorbed to variable degrees by the various tissues and structures they come into contact with. Dense structures absorb more X-rays and appear as bright spots in the final picture [17,18]. Softer, less thick tissues enable more X-rays to flow through, resulting in darker areas. The X-ray imaging process involves three main components: An X-ray source, a patient or object to be imaged and a detector that captures the transmitted X-rays [19,20]. The X-ray machine fires a focused beam of X-rays through the body, which is detected on the opposite side. The detector converts the X-ray energy into electrical signals, which are further processed to create a digital image that displays the attenuation patterns within the body. The working principle of the X-ray machine is further elaborated in Figure 2.
Figure 1:Block diagram of X-ray machine.
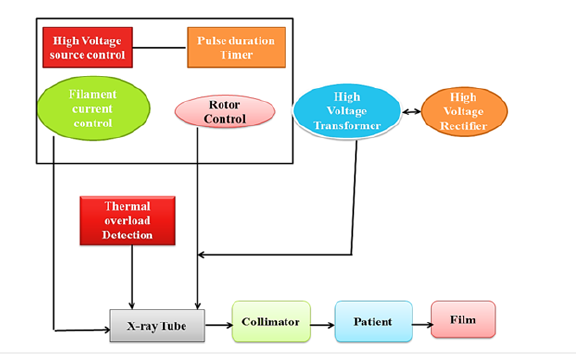
Figure 2:Representation of working principle of X-ray machine.
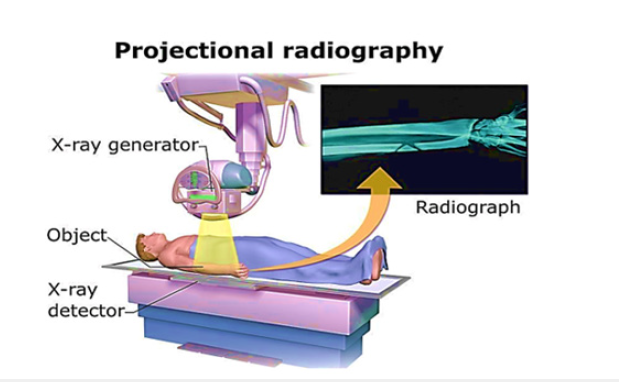
Applications in medical diagnostics and research
Numerous medical diagnostic and research applications exist for X-ray imaging [21]. It is usually utilized to look at lung, dental and gastrointestinal conditions as well as bone fractures. X-ray imaging is essential for the diagnosis and follow-up of conditions including pneumonia, lung cancer, bone fractures, arthritis, dental cavities and gastrointestinal obstructions. X-ray imaging is used for both diagnostic and interventional operations. It directs minimally invasive treatments including biopsies, catheter insertions and angiography. During operations, doctors may see the movement of contrast agents or medical devices inside the body thanks to X-ray fluoroscopy, a real-time X-ray imaging technology. Additionally, X-ray imaging has important uses in research. It aids in the investigation of lung illnesses, skeletal ailments and anatomical variances. A high-resolution X-ray imaging technique called X-ray microtomography makes it possible to see minute structures like bones, tissues and biological samples in three dimensions [22]. Small animal model studies and preclinical research both benefit greatly from this method.
Recent advancements and emerging technologies
Modern improvements in X-ray imaging technology have resulted in better image quality, less radiation exposure and increased diagnostic capabilities. The use of digital detectors instead of conventional film-based X-ray systems in digital radiography has gained widespread acceptance. It has a number of benefits, including the ability to instantly capture photographs, post-process them, send them electronically, make remote consultations easier and archive them. Cone-Beam Computed Tomography (CBCT), which offers three-dimensional imaging capabilities with lower radiation doses than traditional Computed Tomography (CT), is another significant discovery [23]. CBCT is particularly helpful for image-guided procedures and dental and maxillofacial imaging [24].
Additionally, X-ray phase-contrast imaging methods have shown promise as new developments in X-ray imaging. These methods make use of the phase shift that occurs when X-rays travel through various tissues, which improves the contrast of soft tissues and enhances the identification of small abnormalities. Applications for breast, lung and cardiovascular imaging may be possible with X-ray phase-contrast imaging [25]. Amorphous selenium-based and photon-counting detectors, among other developments in X-ray detectors, have enhanced picture quality, increased sensitivity and reduced radiation exposure. In summary, X-ray imaging is a fundamental and popular type of medical imaging. It makes anatomical features visible, helps with disease diagnosis and is essential for controlling interventional Computed Tomography (CT). CBCT imaging is very beneficial in dental and maxillofacial imaging, as well as image-guided procedures [26].
Magnetic Resonance Imaging (MRI)
Principles and basic physics of MRI
Magnetic Resonance Imaging (MRI) is a sophisticated medical imaging technique that creates precise images of the human body using nuclear magnetic resonance principles [27]. It is based on the interaction of atomic nuclei’s magnetic characteristics, notably hydrogen protons, with Radiofrequency (RF) radiation.
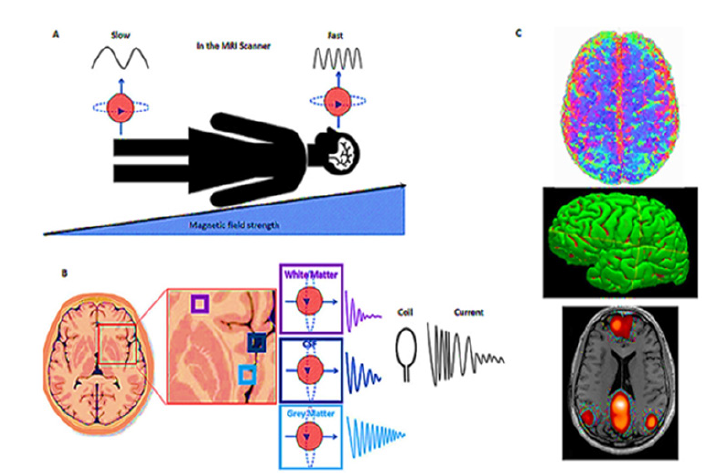
Three key elements make up the fundamental physics of MRI: A powerful magnetic field, RF pulses and a receiver device. A big magnet is used to create a magnetic field around the patient. When exposed to this magnetic field, the protons in the body align with the direction of the field. The protons absorb and release energy as a result of the RF pulses. A three-dimensional image of the internal structures can be constructed by carefully manipulating these RF pulses and analyzing the emitted signals. The working principle of MRI has been further elaborated in Figure 3 [28].
Figure 3:Focusing our imaging. (A) The B1 field is applied, which increases across the body, from foot to head. Hydrogen protons in the head will then be spinning faster than those in the feet. (B) Different tissues, such as white matter, gray matter and Cerebral Spinal Fluid (CSF) in the body give off different amounts of energy. To measure the energy emitted by the protons in the brain when the RF pulse is turned off, we place a coil around the head. (C) This technique can provide many different images of the brain, giving us information about (top right): How the brain is structurally connected via white matter-the information highways of the brain. (Middle right): The volume of gray matter regions of the brain, where information is processed. (Bottom right): How the brain is functionally connectedhow different regions of the brain communicate and work together. This image has been derived from reference [28].
Clinical applications and advantages over other imaging modalities
MRI has a wide range of clinical applications due to its
exceptional soft tissue contrast, multiplanar imaging capabilities
and non-invasive nature [29]. It is particularly valuable in imaging
the brain, spinal cord, joints, muscles and organs in the abdomen
and pelvis. Some common clinical applications of MRI include:
a) Neuroimaging: Using MRI, brain tumors, strokes, multiple
sclerosis, neurodegenerative illnesses and other neurological
problems may be seen and assessed.
b) Musculoskeletal Imaging: MRI is useful for orthopaedic
and sports medicine examinations because it is good at
identifying and evaluating injuries or anomalies in the joints,
tendons, ligaments and bones.
c) MRI offers comprehensive pictures of the liver, kidneys,
pancreas, reproductive organs, and gastrointestinal system,
which aids in the diagnosis and characterization of tumors,
cysts and other abdominal or pelvic disorders.
d) Cardiovascular Imaging: MRI methods like magnetic
resonance angiography (MRA) and cardiac MRI are used to
examine anomalies in blood vessels and monitor cardiac
function.
Being non-ionizing, as opposed to using ionizing radiation like X-ray or CT imaging, is one of the major benefits of MRI. Because of this, MRI can be used to image toddlers, pregnant women, and those who need repeated or ongoing imaging tests. In addition, compared to other imaging modalities, MRI provides good soft tissue contrast resolution, enabling greater visualization of anatomical features and abnormalities [30].
Innovations and developments in MRI technology
MRI technology has progressed via considerable advancements
and changes throughout time, enhancing picture quality, acquisition
speed and patient comfort. Some notable advancements include:
A. High-Field MRI: Increasing the magnetic field strength,
such as with 3 Tesla (3T) MRI systems, enhances the signal-tonoise
ratio and spatial resolution, improving image quality and
diagnostic accuracy.
B. Functional MRI (fMRI): This method maps brain activity
by detecting changes in blood flow and oxygenation, making it
possible to research brain function and connections.
C. Diffusion-weighted imaging (DWI): DWI analyses how
water molecules flow inside tissues to assess tissue architecture
and help diagnose diseases including tumors and stroke.
D. Magnetic Resonance Spectroscopy (MRS): MRS aids
in the identification of cancers and metabolic problems
by determining the concentrations of particular chemical
substances in tissues, which offers metabolic information.
E. Real-time MRI: New developments have made it possible
to dynamically image organs and physiological processes
including speech, swallowing and joint motions.
F. Additionally, shorter scan times, higher image quality,
and overall improved efficiency have been made possible by
advancements in coil design, parallel imaging methods and
image reconstruction algorithms.
To sum up, Magnetic Resonance Imaging (MRI) is a flexible imaging technique that has completely changed the way that doctors diagnose patients. MRI is a vital tool in many medical disciplines thanks to its principles based in nuclear magnetic resonance and its superior soft tissue contrast and multiplanar imaging capabilities. Due to MRI’s benefits over other imaging modalities, namely its non-ionizing properties and enhanced soft tissue visualization, it is often used in clinical settings. The possibilities of MRI technology have also been considerably increased by ongoing advancements. Among the developments that have enhanced picture quality, diagnostic precision, and patient comfort are high-field MRI, functional MRI, diffusion-weighted imaging, magnetic resonance spectroscopy and real-time MRI. MRI is set to play an increasingly important role in precision medicine as research and technical developments proceed, boosting our understanding of complex illnesses and enhancing patient outcomes [31,32].
Ultrasound Imaging
Principles of ultrasound imaging
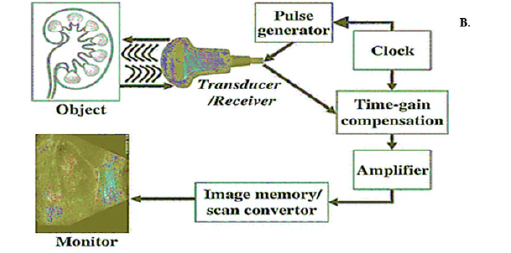
Sonography, another name for ultrasound imaging, employs high-frequency sound waves to provide live pictures of the inside organs. It relies on echoes and reflections of sound waves to function [33]. An ultrasound examination uses a transducer to send ultrasonic waves into the body, where they bounce off of tissues and organs to produce echoes. The transducer gathers these echoes and transforms them into electrical impulses, which are then processed to create images on a display. When an electric current is provided, piezoelectric crystals within the transducer vibrate and produce ultrasonic waves. The frequency of the waves used in ultrasound imaging is typically in the range of 2 to 18 Megahertz (MHz), allowing for the visualization of different tissues and organs at various depths. Higher-frequency ultrasound waves provide better resolution for superficial structures, while lower-frequency waves penetrate deeper into the body. A complete representation of image production by an X-ray machine is shown in Figure 4 [34].
Figure 4:Schematic representation of image production by using an ultrasound machine retrieved from reference [34].
Diagnostic applications and limitations
Ultrasound imaging has many diagnostic applications and is
commonly used in obstetrics, gynecology, cardiology, abdominal
imaging and musculoskeletal examinations. It is a non-invasive
and radiation-free imaging modality, making it safe for adults and
children. Some of the common diagnostic applications of ultrasound
imaging include:
a) Obstetrics: Ultrasound is extensively used for monitoring
fetal development during pregnancy, assessing fetal wellbeing,
detecting abnormalities and guiding procedures such as
amniocentesis.
b) Abdominal Imaging: The liver, gallbladder, kidneys,
pancreas, and spleen are all examined with ultrasound. It can
help discover and diagnose problems including gallstones, liver
tumors and kidney abnormalities.
c) Cardiology: Ultrasound plays a crucial role in evaluating
the structure and function of the heart, assessing heart valve
function, measuring blood flow and detecting abnormalities
such as cardiac tumors or congenital heart defects.
d) Musculoskeletal Imaging: Ultrasound is used to examine
soft tissues, muscles, tendons, ligaments, and joints. It can aid
in the identification of conditions such as sprains, rips and
inflammations.
While ultrasonic imaging has numerous benefits, it also has certain limits. It may not yield as detailed pictures as other imaging modalities such as CT or MRI, particularly in locations where sound waves are dampened, such as deep within the body or behind air-filled structures. Furthermore, ultrasonography is operatordependent since the sonographer’s competence and experience might alter image quality [35]. Patient variables such as body habit or excessive gas can also have an effect on picture quality and diagnostic accuracy.
Emerging trends and improvements in ultrasound technology
Ultrasound technology has advanced significantly, enhancing
picture quality, resolution and usefulness. Among the new trends
and breakthroughs in ultrasound technology are:
A. 3D and 4D Imaging: Three-dimensional (3D) and fourdimensional
(4D) ultrasound technologies provide a volumetric
image of the scanned region, providing for increased visibility
and knowledge of anatomical structures and their evolution.
B. Contrast-Enhanced Ultrasound (CEUS): CEUS is a
technique that uses contrast agents to improve visibility
of blood flow inside organs and tissues. It allows for better
lesion characterization, vascularity measurement and therapy
response tracking.
C. Portable ultrasound equipment: Portable ultrasound
equipment has grown smaller, more affordable, and simpler
to use. In point-of-care settings, emergency medicine and
resource-limited circumstances, these technologies are very
helpful.
D. Fusion Imaging: To give additional information and boost
diagnosis precision, fusion imaging integrates ultrasound with
other imaging modalities like CT or MRI. It enhances lesion
detection, enhances the directing of interventional procedures
and enables more precise treatment planning.
Additionally, improvements in image processing techniques have decreased artefacts and enhanced image quality due to advancements in ultrasound technology. Artificial intelligence and machine learning algorithms are being developed to help with automated image processing, enabling quicker and more precise diagnosis. These new innovations and breakthroughs in ultrasound technology are expanding its applications and elevating it to a more crucial diagnostic tool. Due to their portability, non-invasive imaging capabilities and real-time imaging capabilities, ultrasound technology is particularly flexible and available in a range of healthcare settings. Ultrasound imaging is anticipated to become more important in personalized medicine, telemedicine and the provision of healthcare remotely as technology develops.
Last but not least, sound wave reflection principles are the foundation of the widely used diagnostic procedure known as ultrasonic imaging. It offers a wide range of diagnostic applications in several medical disciplines and stands out for its safety, mobility and real-time imaging capabilities. Despite some of its limitations, ultrasonography is becoming more valuable in clinical settings because of ongoing advancements in the area, such as 3D/4D imaging, contrast-enhanced ultrasound, portable devices and fusion imaging. By combining artificial intelligence and image processing methods, diagnostic efficacy and efficiency are further increased. With continued development, ultrasound imaging has the potential to have a substantial influence on patient care and medical diagnostics [36,37].
Computed Tomography (CT) Imaging
Principles and operation of CT imaging
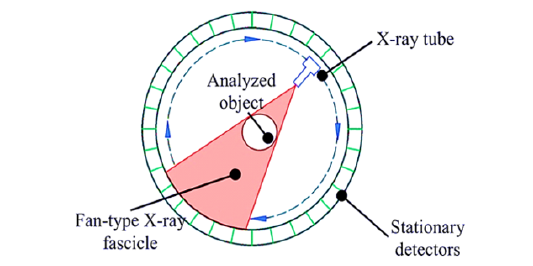
Using X-rays and cutting-edge computer algorithms, the sophisticated medical imaging technique known as Computed Tomography (CT) imaging creates cross-sectional images of the human body [38]. It works on the idea of attenuation, in which X-ray rays travel through the body and are attenuated to various degrees by various tissues. The foundation of CT is an array of detectors and a rotating X-ray source. The gantry, which houses the X-ray tube and detector, is passed by as the patient is lying on a moving table [39]. Several X-ray beams are generated and detected from different angles while the X-ray tube rotates around the subject. The computer processes the released X-rays once the detector establishes their intensity to create cross-sectional images, or “slices,” of the body. A visual representation of the working principle of a CT scan is clear from Figure 5 [40].
Figure 5:Working principle of CT scan an image retrieved from reference number [40].
Clinical applications and benefits
There are many clinical uses for CT imaging, and it has several
advantages for medical diagnosis. Several of the main clinical uses
for CT include:
a) Diagnosis and Staging: CT is often used to diagnose a wide
range of illnesses, including infections, fractures and cancers.
It provides detailed images of buildings’ interiors, enabling
the detection and characterization of irregularities. The use of
CT in cancer staging is also beneficial since it may be used to
assess the amount of tumor involvement and the existence of
metastases.
b) Trauma and Emergency Medicine: CT is essential for
determining the extent of traumatic injuries, especially those
to the head, chest, abdomen, and spine. It gives quick and
accurate pictures that are helpful in identifying and treating
life-threatening illnesses.
c) Vascular Imaging: CT Angiography (CTA), a subtype of CT,
helps to diagnose vascular problems such aneurysms, arterial
stenosis and pulmonary embolism by visualizing blood vessels.
d) Interventional Procedures: For image-guided procedures
including biopsies, drainages and tumor ablations, CT guidance
is employed. Real-time CT imaging enables accurate needle
insertion and process monitoring.
The advantages of CT imaging are its quickness, accessibility and capacity to record fine-grained pictures of both bone and soft tissues. It generates sharp images that may spot minute lesions and subtle irregularities. Additionally, compared to other imaging modalities, CT imaging is less prone to motion artefacts, making it appropriate for individuals who have trouble keeping still throughout the scan [41].
Advances in CT technology and image reconstruction algorithms
CT technological advancements and image reconstruction
techniques have greatly enhanced the quality and efficiency of CT
imaging [42]. Some notable advances include:
A. Multidetector CT (MDCT): By using several detector rows,
MDCT can acquire images more quickly and with better spatial
resolution. In especially for dynamic research, it enables the
collection of smaller slices and increased picture quality.
B. Dual-Energy CT (DECT): DECT uses two different X-ray
energies to learn more about the makeup of the tissues. It
enables material breakdown, permitting improved lesion
characterization, gouty tophi identification and calcium score
evaluation.
C. Iterative reconstruction methods enhance image quality
by cutting down on noise and enhancing contrast. These
algorithms use complex mathematical models to create pictures
from raw data, improving diagnostic precision and lowering
radiation exposure.
D. Cone-Beam CT (CBCT): A two-dimensional detector and a
cone-shaped X-ray beam are used in CBCT imaging, a subset of
CT imaging. It offers finely detailed three-dimensional pictures
for treatment planning and surgical direction, making it very
helpful in dental and maxillofacial imaging.
Additionally, improvements in CT technology have decreased radiation dose by using dose modulation strategies including iterative reconstruction methods and automated exposure management. This maintains picture quality while ensuring patient safety. Using X-rays and sophisticated processing methods, Computed Tomography (CT) imaging creates thorough crosssectional images of the body as a diagnostic tool. It has several practical uses across many medical specialties, facilitating the diagnosis, staging and planning of care for a range of illnesses. Speed, accessibility and the capacity to record high-resolution pictures of both bone structures and soft tissues are just a few advantages of CT imaging. Multidetector, dual-energy and cone-beam CT are recent developments in CT technology that have enhanced picture quality, diagnostic precision and patient safety. Iterative reconstruction techniques have also reduced radiation exposure and enhanced image quality. Medical imaging has significant promise for future breakthroughs as CT technology develops, opening the door for more specialized and individualized patient treatment [43].
Positron Emission Tomography (PET) Imaging
Principles and tracer-based imaging in PET
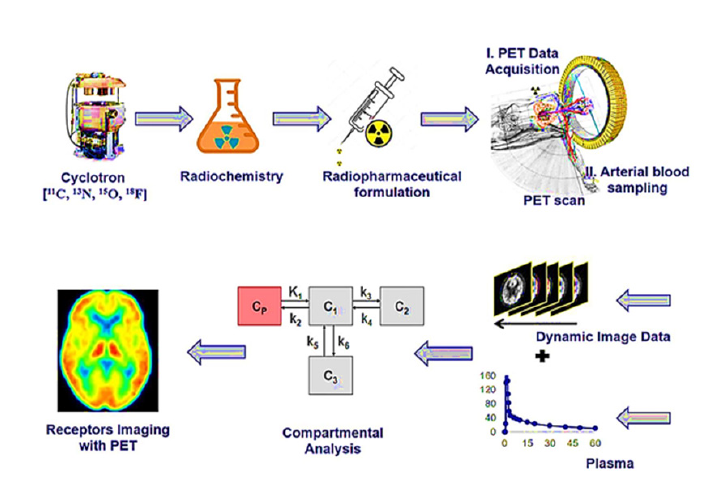
Utilizing positron-emitting radioactive tracers, PET imaging is a molecular imaging technique that shows and quantifies physiological and metabolic processes occurring within the body [11,44]. It is based on nuclear medicine concepts and generates pictures by combining a radioactive tracer with a detection device. During PET imaging, the patient receives a radioactive positronemitting tracer. The tracer undergoes decay and emits positrons, which are positively charged particles. When a positron and an electron collide in the body, they destroy each other and emit two photons in opposing directions. A ring of detectors encircling the patient detects these photons and their location and timing information is utilized to create an image. A complete schematic representation of the whole process is given in Figure 6 [45]. PET imaging is commonly used with various radioactive tracers that target specific biological processes or molecules in the body [46]. For example, Fluorodeoxyglucose (FDG), a commonly used tracer in clinical PET, is taken up by cells in proportion to their glucose metabolism. PET can thus offer information on cellular metabolism and pinpoint areas of enhanced or reduced activity.
Figure 6:The schematic representation of receptor imaging with Positron Emission Tomography (PET) and the flow from radionuclide generation to PET Imaging output, where the initial step is the generation of positronemitting radionuclides, the second step is the radiochemical synthesis, the third step is the preparation of suitable radiopharmaceutical formulation, the fourth step is the PET imaging data acquisition, the fifth step is the PET data pre and post-processing using software technologies, the last stage is the output of PET images reveal physiological changes based on radiotracer uptake corresponding to the intensity and dynamics in the color of images. This image has been retrieved from reference [45].
Clinical applications and challenges
PET imaging has a wide range of clinical applications across
different medical specialties [47]. Some of the key clinical
applications of PET include:
a) Oncology: Planning cancer therapy, staging and diagnosis
depend heavily on PET imaging. It can help identify recurring
cancers and help discover initial tumors. It can also measure
metastatic spread and treatment response.
b) Neurology: In order to diagnose and treat conditions like
Alzheimer’s, Parkinson’s and epilepsy, neurologists employ
PET imaging to measure brain function and metabolism.
c) Cardiology: Myocardial viability and perfusion may be
evaluated with PET, which aids in the diagnosis and evaluation
of coronary artery disease and myocardial infarction. It offers
important details on local blood flow and cardiac metabolism.
The restricted availability of certain radiotracers, the brief halflife of radioactive isotopes and the expensive cost of PET scanners are all problems with PET imaging [48]. In some clinical situations, it may be difficult to interpret PET images since it needs knowledge of nuclear medicine and the underlying illness process.
Recent developments and hybrid imaging techniques (PET/CT, PET/MRI)
The focus of recent developments in PET imaging has been on enhancing image quality, increasing spatial resolution and fusing PET with other imaging modalities. A notable advancement in PET imaging is the combination of Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) to create hybrid imaging systems. Functional and anatomical data from PET and CT are combined in PET/CT. This integration enhances the precision of interpretation and enables exact localization of PET results [49]. Oncology benefits the most from it since it helps with tumor localization, characterization and therapy response evaluation. PET/MRI combines the detailed structural and soft tissue contrast of MRI with the functional and metabolic data of PET. Excellent soft tissue contrast, multi-parametric imaging and simultaneous data gathering are just a few of the special benefits that this hybrid imaging modality offers. It allows for thorough evaluation and accurate localization of abnormalities, which is especially useful in neurology and cancer.
In addition, advances in PET technology have concentrated on enhancing picture quality, lowering radiation dosage, and creating novel radiotracers that focus on certain biological processes. New reconstruction algorithms and image-processing methods are also being developed in an effort to boost diagnostic capabilities and quantitative accuracy [50]. To sum up, PET imaging is an advanced molecular imaging technique that enables the observation and evaluation of biological processes in the body. It has a variety of therapeutic uses in cardiology, neurology and cancer and offers important insights into illness staging, diagnosis, therapy response and patient management. With improvements in radiotracer development, image reconstruction algorithms, and hybrid imaging methods like PET/CT and PET/MRI, PET imaging has continued to advance despite issues with tracer availability and cost. These developments have enhanced spatial resolution, picture quality and the capacity to integrate functional and anatomical data for more precise and thorough diagnostic evaluations. As research and technology advance, PET imaging holds enormous promise for new advancements in personalized medicine, targeted therapy, and understanding complex disorders at the molecular level [51].
Optical Imaging
Fluorescence imaging and bioluminescence imaging: Fundamental optical imaging techniques
Figure 7:Principle of optical imaging A) By administration of probe B) By using receptor gene C) By fluorescence and bioluminescence acquisition. This image has been retrieved from reference number [54].
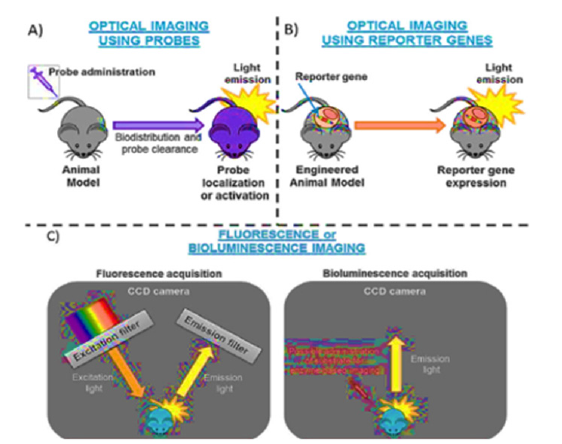
Optical imaging techniques like fluorescence and bioluminescence employ light to monitor and examine biological activities at the molecular and cellular levels [52]. These procedures rely on the production and reception of light signals from specialized reporters or probes. Using fluorescent dyes or probes that produce light of a certain wavelength when stimulated by a light source is necessary for fluorescence imaging [53]. The emitted light is captured by a detector and a picture is generated using the fluorescence intensity. This method enables highly sensitive and precise visualization of certain molecules or cellular structures. Utilizing biological sources like the luciferase enzymes present in fireflies or marine creatures, bioluminescence imaging makes use of the natural light emission from these sources. It is possible to generate and detect bioluminescence signals using specialized cameras by introducing a luciferase reporter gene into cells or animals of interest. With the help of this method, living subjects’ biological processes may be monitored and imaged without any intervention. The principles of optical images have been elaborated in Figure 7 [54].
Applications in preclinical and clinical settings
Numerous uses for optical imaging methods may be found in
both clinical and preclinical settings [55]. A few significant uses are:
A. Optical imaging is frequently used in preclinical research
to investigate a variety of biological processes, including gene
expression, protein-protein interactions, cellular migration
and cancer growth. It offers important insights into the
processes behind illness and potential therapeutic approaches
by enabling researchers to visualize and track molecular and
cellular activities in living animals.
B. Optical imaging methods are employed for cancer
diagnosis, staging, and therapy response monitoring.
Fluorescence imaging has the ability to identify specific cancer
biomarkers, improving surgical guiding and tumor visibility.
Bioluminescence imaging can be used to monitor the growth
and spread of tumors in miniature animal models.
C. Molecular Imaging: Using optical imaging, one may
examine the molecular functions of living things including
enzyme activity, protein-protein interactions and gene
expression [56]. This knowledge is essential for comprehending
disease processes, creating tailored treatments and assessing
therapy effectiveness.
D. In order to examine brain function, neuronal activity,
and neurodegenerative illnesses, optical imaging techniques,
notably fluorescence imaging, are used in neuroscience
research. It enables the real-time observation and tracking of
certain neuronal populations or molecular markers.
Emerging optical imaging technologies and their potential impact
The capabilities of optical imaging technologies continue to
grow, as does the potential influence they might have on clinical
practice and biological research [57]. Upcoming technology and
trends include:
a) A better way to characterize and distinguish between
different tissues and molecular targets is through the use
of multispectral and hyperspectral imaging methods, which
enable the capture of pictures at several wavelengths or small
spectral bands.
b) Photoacoustic Imaging: Combining optical and acoustic
principles, photoacoustic imaging can provide anatomical and
functional data. Based on tissue light absorption, it creates
pictures using laser-induced ultrasonic waves.
c) Nanoparticle-based Imaging: By boosting sensitivity,
target specificity, and multiplexing capacities, nanoparticles,
such as quantum dots and nanoprobes, improve optical imaging.
These nanoparticles can be created to deliver therapeutic drugs
or target certain biomarkers.
d) Optogenetics: Optogenetics uses light-sensitive proteins
to regulate and alter biological activity. It combines genetic
engineering and optical methods. Insights into brain networks
and potential treatments for neurological illnesses are provided
by its exact activation or inhibition of certain cells or circuits.
These new optical imaging technologies have the potential to improve illness diagnosis and treatment monitoring, facilitate personalized medicine strategies and further our knowledge of complicated biological processes. The limited tissue penetration of light, the requirement for specialized equipment and the homogeneity of clinical translation procedures are still issues. We can now see and comprehend biological processes at the molecular and cellular levels thanks to fluorescence and bioluminescence, two optical imaging techniques. These methods give important insights into disease processes, tumor imaging and molecular interactions and have uses in both preclinical research and clinical settings. There is a lot of promise for additional development in the field thanks to the rise of novel optical imaging technologies as multispectral imaging, photoacoustic imaging, nanoparticle-based imaging, and optogenetics. These innovations in sensitivity, specificity and multiplexing pave the way for more precise medical diagnosis, customized treatment regimens, and a deeper comprehension of intricate biological systems [58]. Optical imaging has the potential to advance biological sciences and enhance patient care as research and development proceed.
Molecular Imaging
Introduction to molecular imaging and its role in precision medicine
Figure 8:Schematic representation of different molecular imaging techniques. This image has been retrieved from reference number [59].
A sophisticated imaging technology called molecular imaging enables you to observe and quantify specific molecular and cellular activities in living things. By supplying essential information on the underlying biological mechanisms of disorders, it aids in the development of tailored medical procedures. By focusing on and imaging certain molecules or metabolic pathways, biochemical imaging enables early detection, accurate diagnosis and efficient monitoring of therapeutic response. Molecular imaging techniques include Magnetic Resonance Imaging (MRI), Single-Photon Emission Computed Tomography (SPECT), Positron Emission Tomography (PET) and optical imaging, as shown in Figure 8 [59]. These techniques may identify and map particular molecules, receptors, enzymes and other biological components, revealing information about disease development, therapy efficacy and individual patient features.
Imaging probes and contrast agents for molecular imaging
The imaging probes and contrast chemicals used in molecular imaging are carefully tailored to target certain molecular targets or biological processes of interest [60]. These probes are frequently tagged with radioactive isotopes, fluorescent dyes, or other imaging tags, allowing them to be detected and visualized within the body. PET and SPECT tracers in molecular imaging are radiolabeled compounds that attach to particular receptors or molecules implicated in disease processes. These tracers release gamma rays or positrons, which are detected by specialized imaging devices. MRI contrast agents, on the other hand, take advantage of the interaction between magnetic fields and particular molecules to increase signal intensity while also giving comprehensive anatomical and functional information. In optical molecular imaging, fluorescent dyes and nanoparticles are often utilized, enabling for high-resolution imaging and real-time visualization of molecular targets. These imaging agents can be linked to particular ligands or antibodies, allowing them to attach to disease-related indications [61,62].
Cutting-edge advancements in molecular imaging modalities
Molecular imaging modalities’ capabilities and applicability in
biomedical research and clinical practice have grown dramatically
as a result of technological advancements. Among the significant
advancements are:
a) Multimodal Imaging: By combining several imaging
modalities, such as PET/CT, PET/MRI, and SPECT/CT,
anatomical and functional information is integrated, improving
diagnostic accuracy and allowing for more accurate molecular
target localization.
b) Radiomics and Machine Learning: Radiomics is the
extraction and analysis of large-scale imaging data, including
molecular imaging data, in order to find patterns and extract
relevant information. Machine learning techniques may be used
to these datasets to help with illness categorization, prognosis
and therapy response evaluation.
c) Machine Learning and Radiomics: Radiomics is the
extraction and analysis of large-scale imaging data, particularly
molecular imaging data, to detect patterns and extract useful
information. Machine learning techniques might be used to
these datasets to aid in disease classification, prognosis and
therapeutic response evaluation.
d) Nanotechnology in Molecular Imaging: For their potential
in molecular imaging, nanoparticles such as quantum dots,
magnetic nanoparticles and gold nanoparticles are being
extensively explored. These nanomaterials can be designed
to transport imaging agents, therapeutic medicines, or both,
allowing molecular imaging and treatment.
These groundbreaking advances in molecular imaging modalities have the potential to enhance illness diagnosis, therapy selection, and patient outcomes. They provide for a better knowledge of disease processes, the discovery of novel therapeutic targets and the molecular evaluation of therapy response. However, challenges remain, such as the development of more specific and sensitive imaging agents, imaging method standardization and the implementation of molecular imaging into routine clinical practice. To summaries, molecular imaging is critical in precision medicine because it allows for non-invasive visualization and quantification of specific molecular processes [63]. It provides insight into illness causes, aids in early identification and allows for personalized treatment options. The continual development of imaging probes, contrast chemicals and innovative imaging methodologies positions molecular imaging to benefit.
Image Analysis and Computational Methods
Image analysis and computational techniques’ roles in biomedical imaging
Image analysis and computational approaches are critical for extracting useful information from biological images, allowing for quantitative analysis and assisting in clinical decision-making [64]. These approaches include developing algorithms and software tools to process, analyze and interpret images received through various imaging modalities like MRI, CT, PET and optical imaging. Biomedical imaging creates massive volumes of data, which necessitates sophisticated computational approaches to extract useful information, discover trends and quantify picture properties. Quantitative measurements, geographical localization and morphological characterization of anatomical components, physiological processes and disease indicators are all provided through image analysis techniques [65]. They help in image interpretation, ailment diagnosis, therapy planning and tracking treatment response.
Image segmentation, registration and feature extraction algorithms
Image segmentation is a crucial stage in image analysis in which an image is partitioned into relevant sections or objects [66]. Segmentation algorithms identify boundaries, contours or regions of interest, facilitating the extraction of specific anatomical structures or pathological regions. Various approaches, such as thresholding, region-based methods, edge detection and clustering techniques, are employed for image segmentation. Image registration is another critical process that aligns multiple images or image sequences with facilitating comparison, fusion or tracking of anatomical structures or functional changes over time. Algorithms for image registration strive to align pictures by estimating and optimizing spatial transformations, guaranteeing correct spatial correspondence between images. Feature extraction algorithms capture and quantify relevant information from the segmented or registered images. These algorithms identify distinctive features, such as intensity values, texture, shape or spatial relationships, which are subsequently used for classification, pattern recognition or quantitative analysis. Advanced feature extraction approaches, such as statistical modelling, wavelet analysis and morphological operations, improve the resilience and discriminative capacity of picture analysis [67].
Deep learning and artificial intelligence in biomedical image analysis
Deep learning and Artificial Intelligence (AI) approaches have transformed biomedical image analysis by automating and improving picture interpretation by exploiting big datasets and neural network topologies [68]. Deep learning models used for image analysis include Convolutional Neural Networks (CNNs), Recurrent Neural Networks (RNNs) and Generative Adversarial Networks (GANs). picture classification, object recognition, semantic segmentation, and picture synthesis are all tasks at that deep learning systems Excel at. They learn complex representations and patterns directly from the data, enabling automated identification of abnormalities, accurate segmentation of structures and extraction of relevant image features. Numerous medical imaging applications, including as cancer detection, organ segmentation, illness categorization and therapy response evaluation, have shown that deep learning-based techniques work very well. In artificial intelligence methods for biomedical image analysis, machine learning algorithms including Support Vector Machines (SVM), random forests and ensemble approaches are also utilized [69]. These methods facilitate pattern detection, predictive modelling and feature selection, which enhance diagnosis, prognosis, and individualized treatment plans.
The necessity for huge, annotated datasets, generalizability across various patient groups, interpretability of deep learning models and integration of AI algorithms into healthcare workflows are still issues with image analysis and computational approaches. For better comprehension of anatomical structures, disease processes, and treatment effects, image analysis and computational approaches are essential in biomedical imaging. They offer quantitative measurements, precise segmentation and feature extraction. Deep learning and AI developments have the potential to significantly improve the efficiency, precision and clinical decision-making of image processing in biomedical imaging. Continued image analysis algorithm research and development, as well as the incorporation of AI into medical imaging processes, will further empower clinicians and researchers in their quest for better patient care and medical discoveries.
Challenges and Future Perspectives
Current challenges and limitations in biomedical imaging
Despite the significant advancements in biomedical imaging,
several challenges and limitations persist, hindering its full
potential in healthcare and personalized medicine.
a) Image Quality and Artifacts: Noise, distortions and
artefacts can degrade picture quality and jeopardize diagnostic
and treatment planning accuracy. Technical advancements are
required to improve image acquisition, minimize artefacts and
enhance image resolution and contrast.
b) Radiation Exposure: Modalities such as CT and PET
involve ionizing radiation, which raises concerns about
cumulative radiation exposure in patients. Developing imaging
techniques that reduce radiation dose while maintaining
diagnostic accuracy is a critical challenge.
c) Limited Access to Advanced Imaging: Advanced imaging
modalities, such as PET and MRI, may not be widely available
in all healthcare settings, leading to disparities in patient care.
It is critical to increase access to modern imaging technology to
promote fair healthcare delivery.
d) Data Management and Analysis: Imaging data’s increasing
volume and complexity poses storage, retrieval, and analysis
challenges. To successfully interpret and integrate imaging
data into clinical decision-making, efficient data management
systems, strong image processing algorithms and standardized
methods are required.
Potential solutions and future directions
Addressing the challenges in biomedical imaging requires
continuous research, technological advancements and
collaborations between disciplines. Several potential solutions and
future directions can drive progress in the field:
A. Technological Innovations: Continued development
of imaging hardware and software, including faster image
acquisition, improved image quality and novel imaging
modalities, can overcome current limitations and enhance
diagnostic capabilities.
B. Artificial Intelligence and Machine Learning: Integrating
artificial intelligence and machine learning techniques
into imaging analysis has the potential to automate picture
interpretation, pattern detection and prediction. These
techniques can aid in accurate diagnosis, personalized
treatment planning and predictive assessments.
C. Multimodal Imaging: PET/CT, PET/MRI or hybrid imaging
systems that combine various imaging modalities can give
complementary information and improve diagnostic accuracy.
Integration of imaging data from different modalities can
improve disease characterization and treatment monitoring.
D. Image-guided Interventions: Advancements in imageguided
interventions, such as surgery and targeted therapies,
can improve treatment outcomes and minimize invasiveness.
Real-time imaging during procedures allows for precise
targeting of lesions and minimizes damage to healthy tissues.
Implications for personalized medicine and healthcare
The application of biomedical imaging in personalized medicine
and healthcare is significant [70]:
a) Precision Diagnosis and Treatment: Advanced imaging
techniques enable early sickness detection, detailed disease
characterization and therapeutic response assessment. This
makes it easier to create individualized treatment programmed
based on unique patient features, hence improving therapeutic
outcomes.
b) Patient Stratification and Prognosis: Imaging biomarkers
can help patients stratify by identifying subgroups with
differing illness features or therapy responses. In order to
improve patient outcomes, this permits personalized prognosis
and the choice of effective therapy.
c) Therapeutic Monitoring: Imaging techniques offer a noninvasive
way to keep track of treatment response, enabling
quick adjustments to therapy and assessment of treatment
success. This makes it easier to apply adaptive treatment
techniques and enhances patient care.
d) Biomedical imaging is crucial for drug research and
clinical trials because it permits the assessment of the
pharmacokinetics, target engagement and therapeutic effects
of the drugs. Imaging biomarkers can be employed in clinical
research as substitute endpoints, hastening the discovery of
new drugs.
Biomedical imaging also faces challenges and limitations related to picture quality, radiation exposure, access restrictions and data management. However, technological improvements, such as the incorporation of artificial intelligence, multimodal imaging and image-guided therapies, can help to solve these difficulties. Biomedical imaging holds immense promise for personalized medicine and healthcare, enabling accurate diagnosis, personalized therapy, and improved patient outcomes. Biomedical imaging has the potential to revolutionize healthcare delivery by providing early illness detection, accurate diagnosis and personalized treatment methods by overcoming restrictions and utilizing upcoming technology. Integrating imaging data with other patient information, such as genomes and clinical data, can improve knowledge of disease causes and allow for more focused therapy. Collaboration among researchers, clinicians and industry stakeholders will be critical as the area progresses to promote innovation, optimize imaging techniques and convert improvements into clinical use [71]. Because of ongoing advancements and a focus on problem solving, biomedical imaging is poised to play a significant part in shaping the future of personalized medicine and revolutionizing healthcare for the better.
Conclusion
Recap of key advancements and trends in biomedical imaging
Biomedical imaging has seen extraordinary advances and transformational trends that have revolutionized healthcare and personalized therapy. The development of advanced imaging modalities such as MRI, CT, PET, ultrasound and optical imaging, each with unique capabilities and applications, has been a significant step forward. Integration of image analysis algorithms, artificial intelligence and machine learning approaches has also improved picture interpretation, diagnostic accuracy and predictive modelling. With the introduction of multimodal imaging, image-guided therapeutics and molecular imaging, the breadth of biomedical imaging has expanded, allowing for more thorough and precise illness characterization.
Importance of continued research and collaboration in the field
Continued research and cooperation are critical for biomedical imaging improvement. Discoveries and new solutions to current difficulties may be produced through stimulating multidisciplinary cooperation between scientists, engineers, doctors and industry specialists. Investing in research and development is crucial for enhancing image quality, lowering radiation exposure, improving data processing methods and developing imaging technologies. Furthermore, information, data and best practices must be shared among research institutes and healthcare facilities in order for imaging methods to be widely adopted and standardized.
Potential impact on healthcare and future prospects of biomedical imaging
Biomedical imaging has a substantial and far-reaching influence on healthcare. It has transformed illness diagnosis, treatment planning and therapeutic monitoring, allowing for precision medicine methods that are personalized to unique patient features. Biomedical imaging has the potential to transform healthcare by allowing for earlier disease detection, personalized therapy and improved patient outcomes. Furthermore, combining imaging data with other modalities, such as genomes and clinical data, offers a more complete knowledge of illnesses, allowing for personalized treatment plans and better patient care. Biomedical imaging is predicted to continue developing as a result of technological developments, computational analysis, and artificial intelligence. We foresee the development of increasingly advanced imaging methods with better resolution, sensitivity and accessibility. Furthermore, combining imaging with other developing technologies such as nanotechnology and molecular biology will expand biomedical imaging’s potential. These discoveries will pave the way for novel methods to illness categorization, treatment approaches, and personalized healthcare delivery. Finally, biomedical imaging has transformed healthcare by allowing for accurate diagnosis, targeted therapy and personalized medicine. Continuous cooperation, research, and technical developments in imaging modalities, image processing algorithms and integration with other disciplines will pave the road for even more advances and influence in healthcare.
With the potential to enhance patient care, treatment outcomes and scientific discoveries, biomedical imaging is poised to play a significant role in shaping the future of healthcare and personalized medicine.
References
- Litvinova KS, Rafailov IE, Dunaev AV, Sokolovski SG, Rafailov EU (2017)Non-invasive biomedical research and diagnostics enabled by innovative compact lasers. Progress in Quantum Electronics 56: 1-14.
- Popescu G, Park YK, Choi W, Dasari RR, Feld MS, et al. (2008) Imaging red blood cell dynamics by quantitative phase microscopy. Blood Cells Mol Dis 41(1): 10-16.
- Kateb B, Chiu K, Black KL, Yamamoto V, Khalsa B, et al. (2011) Nanoplatforms for constructing new approaches to cancer treatment, imaging and drug delivery: What should be the policy? Neuroimage 54: S106-S124.
- Hsu JC, Nieves LM, Betzer O, Sadan T, Noël PB, et al. (2020) Nanoparticle contrast agents for X‐ray imaging applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol 12(6): e1642.
- Biteen JS, Blainey PC, Cardon ZG, Chun M, Church GM, et al. (2016) Tools for the microbiome: Nano and beyond. ACS Nano 10(1): 6-37.
- Zhang W, Hongrui L, Yongqin L, Hanlong L, Yumin C (2021) Application of deep learning algorithms in geotechnical engineering: A short critical review. Artificial Intelligence Review 54(8): 5633-5673.
- Zhao Z, Zeng Z, Xu K, Chen C, Guan C (2021) DSAL: Deeply supervised active learning from strong and weak labelers for biomedical image segmentation. IEEE J Biomed Health Inform 25(10): 3744-3751.
- Low LA, Mummery C, Berridge BR, Austin CP, Tagle DA (2021) Organs-on-chips: Into the next decade. Nat Rev Drug Discov 20(5): 345-361.
- Roco MC, Bainbridge WS (2002) Converging technologies for improving human performance: Integrating from the nanoscale. Journal of nanoparticle research 4: 281-295.
- Als-Nielsen J, Morrow DM (20011) Elements of modern X-ray physics. John Wiley & Sons.
- Massoud TF, Gambhir SS (2003) Molecular imaging in living subjects: Seeing fundamental biological processes in a new light. Genes Dev 17(5): 545-580.
- Venkatasubramanian V, Rengaswamy R, Yin K, Kavuri SN (2003) A review of process fault detection and diagnosis: Part I: Quantitative model-based methods. Computers & chemical engineering 27(3): 293-311.
- Webb A (2022) Introduction to biomedical imaging. John Wiley & Sons.
- Sakamoto JH, Ven AL, Godin B, Blanco E, Serda RE, et al. (2010) Enabling individualized therapy through nanotechnology. Pharmacol Res 62(2): 57-89.
- Eslami M, Tabarestani S, Albarqouni S, Adeli E, Navab N, et al. (2020) Image-to-images translation for multi-task organ segmentation and bone suppression in chest x-ray radiography. IEEE transactions on medical imaging 39(7): 2553-2565.
- Vollmer M (2021) Physics of the electromagnetic spectrum. In: López VM, Bhat R (Eds.), Electromagnetic Technologies in Food Science. (1st edn), Wiley Online Library, New Jersey, USA, pp. 1-32.
- Henderson R (1995) The potential and limitations of neutrons, electrons and X-rays for atomic resolution microscopy of unstained biological molecules. Q Rev Biophys 28(2): 171-193.
- Ilyas M, Muhammad S, Iqbal J, Amin S, Al-Sehemi AG, et al. (2022) Insighting isatin derivatives as potential antiviral agents against NSP3 of COVID-19. Chem Zvesti 76(10): 6271-6285.
- Seibert JA, Boone JM (2005) X-ray imaging physics for nuclear medicine technologists. Part 2: X-ray interactions and image formation. J Nucl Med Technol 33(1): 3-18.
- Ilyas M, Ayu AR, Shehzad RA, Khan MA, Perveen M, et al. (2022) A DFT approach for finding therapeutic potential of two dimensional (2D) Graphitic Carbon Nitride (GCN) as a drug delivery carrier for curcumin to treat cardiovascular diseases. Journal of Molecular Structure 1257: 132547.
- Nayak SR, Nayak DR, Sinha U, Arora V, Pachori RB (2021) Application of deep learning techniques for detection of COVID-19 cases using chest X-ray images: A comprehensive study. Biomed Signal Process Control 64: 102365.
- Mizutani R, Suzuki Y (2012) X-ray microtomography in biology. Micron 43(2-3): 104-115.
- Kau C, Richmond S, Palomo JM, Hans MG (2005) Three-dimensional cone beam computerized tomography in orthodontics. J Orthod 32(4): 282-293.
- Eggers G, Mühling J, Hofele C (2009) Clinical use of navigation based on cone-beam computer tomography in maxillofacial surgery. British Journal of Oral and Maxillofacial Surgery 47(6): 450-454.
- Kwan AC, Pourmorteza A, Stutman D, Bluemke DA, Lima JA (2021) Next-generation hardware advances in CT: Cardiac applications. Radiology 298(1): 3-17.
- Jahandideh H, Yarahmadi A, Rajaieh S, Shirazi AO, Milanifard M et al. (2020) Cone-beam computed tomography guidance in functional endoscopic sinus surgery: A retrospective cohort study. Journal of Pharmaceutical Research International 31(6): 1-7.
- Odéen H, Parker DL (2019) Magnetic resonance thermometry and its biological applications-physical principles and practical considerations. Prog Nucl Magn Reson Spectrosc 110: 34-61.
- Broadhouse K (2020) The physics of MRI and how we use it to reveal the mysteries of the mind. Frontiers for Young Minds 7: 23.
- Marciani L (2011) Assessment of gastrointestinal motor functions by MRI: A comprehensive review. Neurogastroenterol Motil 23(5): 399-407.
- Horng A, Brun E, Mittone A, Gasilov S, Weber L, et al. (2014) Cartilage and soft tissue imaging using X-rays: Propagation-based phase-contrast computed tomography of the human knee in comparison with clinical imaging techniques and histology. Invest Radiol 49(9): 627-634.
- MacEachern SJ, Forkert ND (2021) Machine learning for precision medicine. Genome 64(4): 416-425.
- Muhammad S, Amin S, Iqbal J, Al-Sehemi AG, Alarfaji SS, et al. (2022) Insighting the therapeutic potential of fifty (50) shogaol derivatives against Mpro of SARS-CoV-2. Journal of Computational Biophysics and Chemistry 21(5): 555-568.
- Szabo TL (2014) Diagnostic ultrasound imaging: Inside out. In: Szabo TL (Ed.), (2nd edn), Academic press., Cambridge, Massachusetts, USA.
- Fulgham PF (2021) Physical principles of ultrasound. Practical Urological Ultrasound 13-30.
- Gardner CJ, Brown S, Ansert SH, Harrigan P, Kisslo J, et al. (1992) Guidelines for cardiac sonographer education: Report of the American society of echocardiography sonographer education and training committee. J Am Soc Echocardiogr 5(6): 635-639.
- Barnett SB, Haar GR, Ziskin MC, Rott HD, Duck FA, et al. (2000) International recommendations and guidelines for the safe use of diagnostic ultrasound in medicine. Ultrasound in Medicine & Biology 26(3): 355-366.
- Saba A, Sarwar F, Muhammad S, Ilyas M, Iqbal J, et al. (2022) Insighting the inhibitory potential of novel modafinil drug derivatives against estrogen alpha (ERα) of breast cancer through a triple hybrid computational methodology. Journal of molecular liquids 366: 120234.
- Abdallah YM, Alqahtani T (2019) Research in medical imaging using image processing techniques. Medical Imaging-Principles and Applications 10.
- Goldman LW (2007) Principles of CT and CT technology. J Nucl Med Technol 35(3): 115-128.
- Tudor DI, Pastrama SD, Hadar A (2014) The use of computed tomography and ultrasonic imaging for assessment of defects in plates made of a polyesteric resin. Engineering Transactions 62(1): 17-31.
- Hallowell LM, Stewart SE, Silva CT, Ditchfield MR (2008) Reviewing the process of preparing children for MRI. Pediatr Radiol 38(3): 271-279.
- Flohr TG, Schaller S, Stierstorfer K, Bruder H, Ohnesorge BM, et al. (2005) Multi-detector row CT systems and image-reconstruction techniques. Radiology 235(3): 756-773.
- Ahmad Z, Rahim S, Zubair M, Ghafar JA (2021) Artificial intelligence (AI) in medicine, current applications and future role with special emphasis on its potential and promise in pathology: Present and future impact, obstacles including costs and acceptance among pathologists, practical and philosophical considerations. A comprehensive review. Diagn Pathol 16(1): 24.
- Khan MA, Iqbal J, Ilyas M, Ayub AR, Zhu Y, et al. (2022) Controlled supramolecular interaction to enhance the bioavailability of hesperetin to targeted cancer cells through graphyne: A comprehensive in silico study. RSC Adv 12(10): 6336-6346.
- Nerella SG, Singh P, Sanam T, Digwal CS (2022) PET molecular imaging in drug development: The Imaging and chemistry perspective. Front Med (Lausanne) 9: 812270.
- Pimlott SL, Sutherland A (2011) Molecular tracers for the PET and SPECT imaging of disease. Chemical Society Reviews 40(1): 149-162.
- Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, et al. (2000) A combined PET/CT scanner for clinical oncology. J Nucl Med 41(8): 1369-1379.
- Fowler JS, Wolf AP (1997) Working against time: Rapid radiotracer synthesis and imaging the human brain. Accounts of Chemical Research 30(4): 181-188.
- Sapir EE, Keidar Z, Shalom RB (2009) Hybrid imaging (SPECT/CT and PET/CT)-improving the diagnostic accuracy of functional/metabolic and anatomic imaging. Semin Nucl Med 39(4): 264-275.
- Avanzo M, Wei L, Stancanello J, Vallières M, Rao A, et al. (2020) Machine and deep learning methods for radiomics. Med Phys 47(5): e185-e202.
- Ginsburg GS, McCarthy JJ (2001) Personalized medicine: Revolutionizing drug discovery and patient care. Trends Biotechnol 19(12): 491-496.
- Bhaumik S, Gambhir SS (2002) Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc Natl Acad Sci 99(1): 377-382.
- Lichtman JW, Conchello JA (2005) Fluorescence microscopy. Nature methods 2(12): 910-919.
- Martelli C, Dico AL, Diceglie C, Lucignani G, Ottobrini L (2016) Optical imaging probes in oncology. Oncotarget 7(30): 48753-48787.
- Angelo JP, Chen SJ, Ochoa M, Sunar U, Gioux S, et al. (2019) Review of structured light in diffuse optical imaging. J Biomed Opt 24(7): 1-20.
- Massoud TF, Paulmurugan R, Abhijit De, Ray P, Gambhir SS (2007) Reporter gene imaging of protein-protein interactions in living subjects. Curr Opin Biotechnol 18(1): 31-37.
- Ayaz H, Baker WB, Blaney G, Boas DA, Bortfeld H, et al. (2022) Optical imaging and spectroscopy for the study of the human brain: Status report. Neurophotonics 9(S2): S24001.
- He X, Liu X, Zuo F, Shi H, Jing J (2023) Artificial intelligence-based multi-omics analysis fuels cancer precision medicine. Semin Cancer Biol 88: 187-200.
- Morato YL, Paredes KO, Chamizo LL, Marciello M, Filice M (2021) Recent advances in multimodal molecular imaging of cancer mediated by hybrid magnetic nanoparticles. Polymers 13(17): 2989.
- Dammes N, Peer D (2020) Monoclonal antibody-based molecular imaging strategies and theranostic opportunities. Theranostics 10(2): 938-955.
- Sedgwick AC, Harvey P, Iovan DA, Smith G, He XP, et al. (2020) Metal-based imaging agents: Progress towards interrogating neurodegenerative disease. Chemical Society Reviews 49(10): 2886-2915.
- Ayub AR, Kalsoom S, Ayub AR, Ilyas M, Hassan N, et al. (2023) Host-guest coupling to potentially increase the bio-accessibility of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea by nanocarrier graphyne for brain tumor therapy, a comprehensive quantum mechanics study. J Mol Graph Model 123: 108517.
- Salih S, Elliyanti A, Alkatheeri A, Yafei FA, Almarri B, et al. (2023) The role of molecular imaging in personalized medicine. J Pers Med 13(2): 369.
- Jayatilake SM, Ganegoda GU (2021) Involvement of machine learning tools in healthcare decision making. J Healthc Eng 2021: 6679512.
- Timmeren JE, Cester D, Lang ST, Alkadhi H, Baessler B (2020) Radiomics in medical imaging-“how-to” guide and critical reflection. Insights Imaging 11(1): 1-16.
- Hossain MD, Chen D (2019) Segmentation for Object-Based Image Analysis (OBIA): A review of algorithms and challenges from remote sensing perspective. ISPRS Journal of Photogrammetry and Remote Sensing 150: 115-134.
- Mordia R, Verma AK (2022) Visual techniques for defects detection in steel products: A comparative study. Engineering Failure Analysis 134: 106047.
- Suganyadevi S, Seethalakshmi V, Balasamy K (2022) A review on deep learning in medical image analysis. Int J Multimed Inf Retr 11(1): 19-38.
- Nasir N, Kansal A, Alshaltone O, Barneih F, Sameer M, et al. (2022) Water quality classification using machine learning algorithms. Journal of Water Process Engineering 48: 102920.
- Berg AV, Mummery CL, Passier R, Meer AD (2019) Personalised organs-on-chips: Functional testing for precision medicine. Lab Chip 19(2): 198-205.
- Allen B, Seltzer SE, Langlotz CP, Dreyer KP, Summers RM, et al. (2019) A road map for translational research on artificial intelligence in medical imaging: From the 2018 National Institutes of Health/RSNA/ACR/The academy workshop. J Am Coll Radiol 16(9): 1179-1189.
© 2023 Maroof Ahmad Khan, This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






