- Submissions

Full Text
Significances of Bioengineering & Biosciences
Investigating the Potential of Indigenous Soil Bacteria for Enhancing Thermal Conductivity: A Study on Improving Soil Heat Transfer Properties
Shivani Mistry1*, Manish Shah1, Shalini Singh1 and Rakeshkumar Panchal2
1Department of Applied Mechanics, L.D. College of Engineering, India
2Department of Microbiology and Biotechnology, Gujarat University, India
*Corresponding author:Shivani Mistry, Department of Applied Mechanics, L.D. College of Engineering, India
Submission: June 07, 2023 Published: June 22, 2023

ISSN 2637-8078Volume6 Issue2
Abstract
The thermal conductivity of soil is a crucial parameter for designing energy geo-structures, as it determines the rate at which heat can be transferred through a material. This paper explores the influence of the presence of bacteria on soil thermal conductivity. To investigate this, soil samples were collected from four different regions in Gujarat, including Surat, Bhadbhut, Navsari, and Bhuj. A laboratory-scale experimental setup was developed based on the guarded hot plate method to measure the thermal conductivity of soil samples. The microorganisms were isolated from the soil samples using a serial dilution and spread plate method and two types of microorganisms were selected from each soil sample. The isolated microorganisms were introduced into the soil samples and the thermal conductivity was measured after a week of incubation. The thermal conductivity of soil samples was measured for curing periods of 7 days, and the results of thermal conductivity were compared before and after treatment. The results demonstrated that E. coli decreases the thermal conductivity values of sandy soil and S. aureus gives good results for sandy soil compared to clayey soil. The microorganisms isolated from soil were all of the Bacillus family.
Keywords: Thermal conductivity; Micro-organisms; Temperature; Guarded hot plate method; Bacillus
Introduction
The thermal conductivity of soil is a crucial parameter for designing energy geostructures, as it determines the rate at which heat can be transferred through a material. It is measured in units of Watts Per Meter Kelvin (W/mK). To explore the effect of microbiology and biotechnology on thermal conductivity, this research project is multidisciplinary in nature, incorporating geotechnical engineering with microbiology. The research involves inducing microbes in different types of soil to observe changes in thermal conductivity over time. The soil is first treated with microbes, which transform it into a microbiologically engineered material, and changes in thermal conductivity are monitored. Soils, as observed before, are “particulate media”, i.e., substances having a skeleton of easily separable particles which enclose interconnected and irregularly shaped voids. The void space may be wholly or partly filled with a liquid, generally water containing salts in solution [1]. Thermal properties of soil refer to the characteristics of soil that are related to its ability to conduct, store and transfer heat. Soil thermal properties are important in many agriculture, engineering and meteorological applications. It involves Heat Capacity (C), Thermal Diffusivity (α) and Thermal Conductivity (k) [2]. These properties are also important in engineering applications such as the electric current rating for buried cables depends on the thermal conductivity of the surrounding soil as does the efficiency of heat pump systems [2]. Soil thermal conductivity is important in determining the effect of cold and frost on soil used as foundation material for roads, airfields, pipelines and buildings in cold regions [3].
The soil possesses a certain distribution of grain sizes and shapes, which determines its density, porosity and pore size distribution [3,4]. The variables that affect soil thermal conductivity can be categorized into two main categories: those that are inherent to the soil and those that can be managed or controlled. The texture and mineral composition of the soil are a couple of those elements or characteristics that are inherent to the soil itself [5]. Factors influencing a soil’s thermal conductivity that can be managed externally include water content and soil management. The thermal conductivity of soil depends on a number of factors, such as density, moisture content, organic matter and salt content [4]. For the soil types of sand, sandy loam, loam and clay loam, thermal conductivity increases with an increase in soil density and moisture content. Also, thermal conductivity decreases with an increase in the amount of added salt. Sand has higher values of thermal conductivity than clayey soil for the same salt content. An increase in dry density will result in an increase in the thermal conductivity of the soils due to an increase in volume, which will result in an increase in particle contact and a decrease in air volume [6].
The thermal conductivity of materials can be measured by two methods: steady-state methods and transient methods. Steady state conditions refer to a constant temperature at each point of the sample, i.e., not a function of time. Transient methods are used to record measurements during the process of heating up or cooling down a material or fluid. These methods have the advantage of giving quicker measurements than steady-state methods. Steadystate methods involve the guarded hot plate method, the axial flow method, the cylinder method and the heat flow meter method. The hot-wire method, needle probe method, and transient plane source method are included in transient methods [7-9]. The choice of method depends on several factors, such as the accuracy desired, the temperature range to be covered, the kind of specimen, the total cost, fabrication, etc. Among the absolute methods, the guarded hot plate is the most widely used and precise method for determining the thermal conductivity of materials and has been adopted by the American Society for Testing and Materials as a standard method [10] Attached to the plates [9].
The guarded hot plate method involves placing the specimen between two plates. Power is supplied to the heater and heat flows axially through the specimen. The temperature difference between two plates is then measured using a thermocouple or thermistors that are thermal needle probe method involves inserting a heating element and a temperature measuring element into the soil sample. A known voltage and current are applied and the rise in temperature over time is recorded. Based on this data, the thermal conductivity of soil can be determined [9]. The accuracy of the thermal conductivity values obtained through guarded hot plate apparatus is affected by temperature, which can be eliminated by placing the thermocouple in an appropriate position [10]. In addition to the traditional laboratory methods for measuring soil thermal conductivity, new techniques have been developed, such as the thermal cell and spherical probe. These methods allow for the testing of two soil samples simultaneously and are known to provide reliable results [11,12]. When comparing laboratory and in-situ methods for measuring soil thermal conductivity, the needle probe (laboratory method) is faster but yields lower thermal conductivity values. On the other hand, the thermal cell (field method) is more time-consuming but provides more accurate results [13].
To predict thermal conductivity values, several thermal conductivity models have been developed. Many soils thermal conductivity models have been developed in the literature [14-22]. Johansen O [15] proposed the normalized Thermal Conductivity (kr) concept, which has been used to study the effects of soil type, Porosity (n), Degree of Saturation (Sr) and mineralogical components on soil thermal conductivity in a unique way through the kr-Sr relationship. Other thermal conductivity models have made extensive use of this idea. For example, Cote J et al. [16] proposed a generalized thermal conductivity model for soils and construction materials. Zhang N et al. [23] modified the Cote J et al. [16] model for sands with high quartz content. A comprehensive review of soil thermal conductivity models can be found in Zhang N et al. [22] study.
By combining biological processes, a new technique for improving soil qualities in geotechnical engineering has been created. Microbial-Induced Calcite Precipitation (MICP) is one such biological technique that is gaining popularity due to its sustainable and effective approach to soil improvement [24-26]. This technique is based on the hydrolysis of urea, which is made possible by bacterially generated urease enzymes. In the presence of water, the hydrolysis of urea breaks down into ammonium ions and carbonate ions [27]. Calcium carbonate (also known as calcite), which is created when the carbonate ions combine with the calcium ions, serves as a binding agent for the soil. It is predicted that soils treated with MICP will have less porosity, which will lead to less permeability. As a result, the flow of water in the pore space of soils will be affected [28]. It was also found that the permeability decreased by an order of magnitude after one cycle of MICP treatment [29]. Additionally, the unconfined compressive strength of MICP-treated sands was significantly improved [30]. Paassen LA et al. [31] proved the feasibility of using the MICP technique to reinforce soils based on a series of large-scale laboratory tests. The main advantage of the technique is that it can replace cementitious materials for reinforcing soils without causing a potential detrimental impact on the natural environment. The aim of this study is to observe the changes in the thermal conductivity values by measuring them experimentally through a set-up developed in the laboratory based on the guarded hot plate method.
Material and Methods
Soil specimen
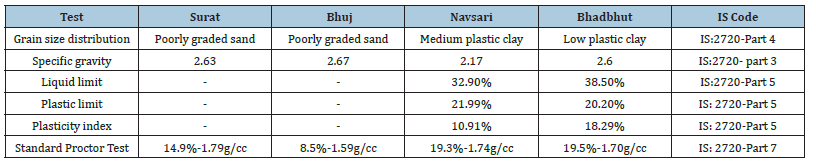
The four types of soil employed in this study were obtained from the different regions of Gujarat, India. Four regions were taken: Surat, Navsari, Bhadbhut and Bhuj. Index property tests such as grain size distribution, specific gravity, Atterberg’s limit and standard proctor tests were performed in the laboratory according to Indian standard specifications. Table 1 tabulates the values of the physical properties of soil. Soil from Surat and Bhuj regions was classified as poorly graded sand, while soil from Navsari, Dholera and Bhadbhut had medium plastic clay and low plastic clay. To investigate the effectiveness of the biological treatment for different types of soil, the specimens were compacted into 80% of MDD.
Table 1:Physical properties of soil.
Microorganism
The microorganisms used in this study were isolated from the soil. The bacterial colonies were isolated using a process called isolation and screening. Two bacterial strains that were isolated from the soil were kept unknown while introducing them into the soil and three known bacterial strains were used. Bacteria were isolated using the serial dilution method, in which 1g of soil was taken in a sterile test tube containing 10ml of distilled water. Mix the sample by shaking it vigorously so that organisms are distributed uniformly throughout the sample. Carry out the following procedure under aseptic conditions between burners: With the help of a sterile pipette, remove 1ml of the original sample and deliver it into the first dilution blank containing 9ml of sterile distilled water. This gives a dilution of 10 (1/10). Mix the 10 dilutions well, and using another sterile pipette, remove 1ml of the 10 dilution and deliver it to another dilution blank containing 9ml of sterile distilled water.
This gives a dilution of 10 (1/100). Continue further in a similar manner until the desired dilutions are made. Then 100L of water from every dilution of soil sample was spread on Nutrient Agar Base Petri Plates. Petri plates were incubated at 37 °C for 24-48 hours (Anyadoh et al. 2017). Isolated colonies of bacteria present in the soil sample were obtained. The colonies were counted, and cfu/ml were counted and noted down. Colony characteristics of dominant colonies were observed and noted down. Various isolates were subculture to get a pure culture of each isolate by the fourflame method and slants of each pure culture were also prepared and preserved at 4 °C for testing the thermal conductivity (Anitha et al. 2018). Two dominant bacterial strains were selected from each of the six soils.
Test setup
Figure 1:Thermal conductivity measuring instrument.
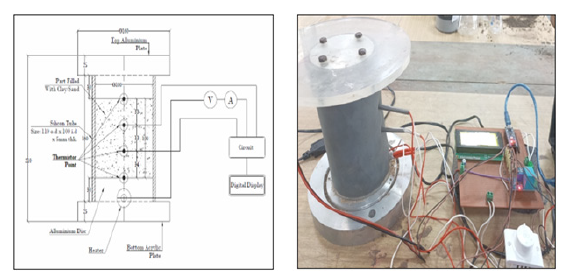
The design is based on the application of Fourier’s law of one-dimensional heat conduction under steady state condition. According to the definition of the one-dimensional steady state, heat flow is supposed to be in one direction, implying that no lateral heat transfer occurs within the specimen. Practically, this condition is difficult to attain due to ambient temperature interference, which provides an extra radial temperature dispersion to the necessary axial temperature gradient. It is essential to consider the effect of longitudinal and radial heat flow. The common method to minimize the effect of ambient temperature is to use an insulation layer. The device includes two plates i.e., top plate and bottom plate. Dimensions are displayed in Figure 1. Top plate is made of acrylic and bottom plate is made of aluminum. Both plates are connected to a readout unit which controls the temperature. A thermal jacket is placed around the sample which serves as a heat insulation barrier. The temperature sensors are provided at top and bottom plates also two sensors are provided at 2/4th and 3/4th distance from the bottom plates to measure the temperature gradient. The temperature sensors provided at top and bottom are separated at distance L from each other and connected with the readout unit. The readout unit consists of a dimmer, voltmeter and ammeter. Since the test is based on the steady state condition, the test is continued until the steady state condition is achieved. The thermal conductivity k can be calculated from the following expression.
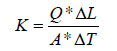
Where,
k = Thermal conductivity of soil
Q = V * I = The amount of heat passing through a cross-section A
V = Voltage applied to heater
I = Current applied to the heater
A = Cross sectional area of specimen
ΔT= T1-T2 = Temperature difference
T1 = Temperature of bottom plate
T2 = Temperature stabilized at top plate
ΔL= Length of the specimen
The obtained results concerning the untreated soil samples are validated using Karsten’s empirical equations for clayey and sandy soils.
a) Clayey soils:
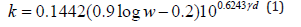
b) Sandy soils:
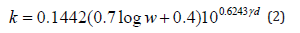
Where k in W/m K
ɣd in g/cc
w in %
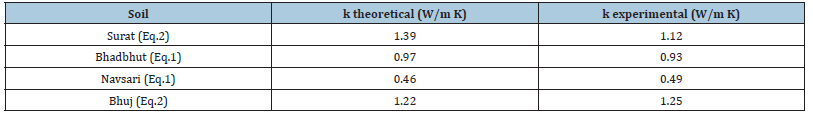
Table 2 shows the theoretical and experimental values of thermal conductivity which reflects no major changes. So, the set can be used for measurement of thermal conductivity of soil.
Table 2:Physical properties of soil.
Specimen preparation
A specimen mould is made using split PVC pipe of 100mm diameter and 100mm length. The pipe is attached by adhesive tape. Sterile distilled water is used for the preparation of the bacterial solution that was added to the soil. The soil mixed with the solution is compacted into the PVC mould at 80% of the MDD. First, the soil is oven-dried for the sample preparation. Soil is weighed as required and bacterial broth is prepared by inducing bacteria into the nutrient media that was mixed with the soil. The mixed soil is then added to the PVC mould and compacted. The sample is then incubated for one week at room temperature.
Thermal conductivity test
The thermal conductivity of the prepared samples was tested with a thermal conductivity measuring instrument. The bacteria selected from different soil samples are: Surat (S1, S2), Navsari (N1, N2), Bhadbhut (BB1, BB2) and Bhuj (B1, B2). Two different types of bacteria isolated from each of six types of soil were induced into the soil samples, which were kept unknown. While two bacteria, namely E. coli and Staphylococcus aureus, were added to all types of soils. A total of four samples of each soil type were made, adding various bacteria. One test took six hours to complete. The readings were noted, manual calculations were done, and reports were prepared. The results were then analyzed.
Experimental Results and Discussion
Total viable plate count
Three N agar plates of dilution 10-2 , 10-3 and 10-4 for soil sample are put for 24 to 48 hours for the growth of colonies and total number of colonies are counted.
Isolation of dominant bacteria
After 24-48 hours of incubation at 372 °C, total colonies were counted, and dominant colonies were picked from each soil sample on the nutrient agar plate. From all 16 isolates, based on their growth, two isolates from each soil sample are selected for the test of thermal conductivity. Based on their growth in slant grading, eight isolates are selected for further study and analysis. Isolates from the Surat site were named S1, S2, for Bhadbhut it was BH1, Bh2, for the Navsari site N1, N2, and isolates from the Bhuj site were named B1, B2.
Colony characteristics of isolates: Selected 8 isolates are cultured separately on N. agar plates, and their morphological studies are done under a microscope.
Thermal conductivity test results
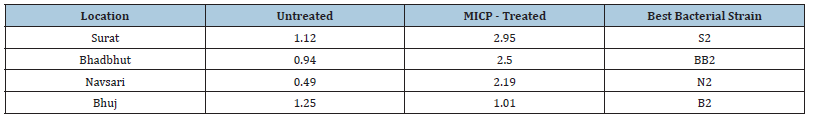
Table 3 summarizes the results obtained for the k values with respect to bacterial strain of untreated and MICP treated soil. As mentioned above, Table 4 shows the summary of thermal conductivity values increment and decrement due to corresponding bacteria (Table 5). The increment of k value in Surat, Bhadbhut and Navsari is due to unknown internal strains, namely S2, BB2 and N2, respectively, whereas in Madhavpur and Dholera it is due to E. coli and B. subtilis, respectively (Figure 2 & 3). In Bhuj soil, the thermal conductivity decreases when the B2 bacterial strain is added. Figure 4 Shows the comparison of thermal conductivity of all the various soil w.r.t. external bacterial strains. It indicates that E coli is more dominant in Bhadbhut. soil which is low plastic clay and it decreases the k value in Surat soil which is poorly graded sand. Whereas talking about S. aureus results show that k value is higher in Bhuj soil which is poorly graded sand and it is lower in Navsari soil which is medium plastic clay.
Figure 2:Thermal conductivity of Surat and Bhadbhut.
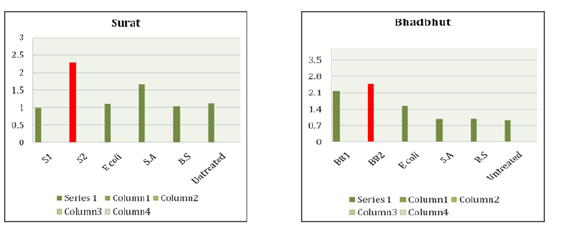
Figure 3:Thermal conductivity of Navsari and Bhuj.
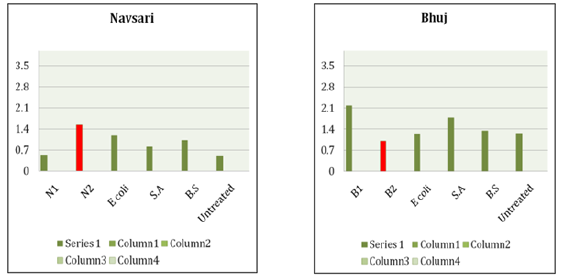
Figure 4:Comparison of k values of different soil types w.r.t. external bacterial strain.
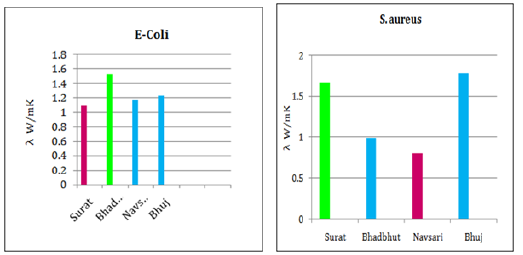
Table 3:Total viable counts of colonies on N agar plate.
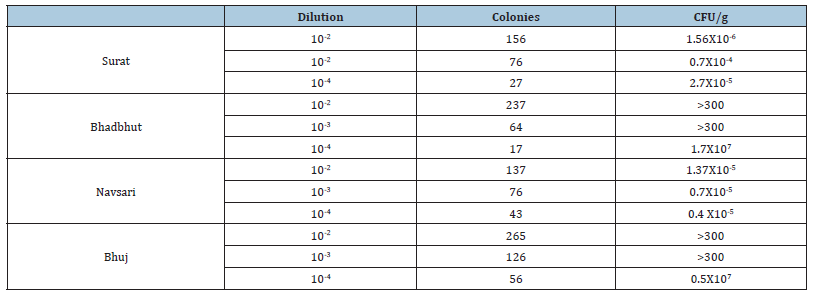
Table 4:Colony characteristics of isolates.
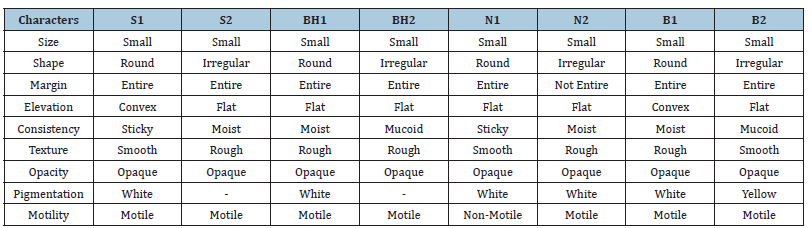
Table 5:Summary of thermal conductivity of untreated and MICP - treated soil and bacterial strain.
Identification of the bacteria
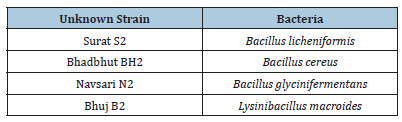
After analysis of the results, it becomes necessary to identify the unknown bacterial strain which helped in enhancing the thermal conductivity of soil. The bacterial strains were then sent for identification. The following table shows the identified bacterial strains (Table 6).
Table 6:Identification of unknown bacterial strain.
Conclusion
For soil samples from Bhadbhut and Navsari, eight dominant bacterial colonies were isolated and used for the measurement of the thermal conductivity of clay soil. Bacteria were isolated from soil samples, and pure cultures of isolates were obtained. And for soil samples from Surat and Bhuj, four dominant bacterial colonies were isolated and used in the measurement of thermal conductivity of sandy soil. From the microscopic observation of Gram staining, it was found that S1, S2 and B1 and B2 isolates were Gram-positive cocci, motile, and non-capsule formers.
The tests have confirmed that the thermal conductivity varies with different test bacteria. Isolates of BH2 in Bhadbhut, N2 in Navsari, S2 in Surat and B2 in Bhuj gave the best results. After the identification, it was found that S2 is Bacillus licheniformis, which turns out to be the most potent bioagent, with a maximum amount of CaCO3 precipitate of 3.14g/100 mL in the optimal conditions [32]. BH2 found as the local Bacillus cereus strain showed high urease activity and could be a viable and economical solution where hot and humid climatic conditions are encountered [33]. N2 and B2 were found as Bacillus glycinifermentans and Lysinibacillus macroides respectively. All the bacteria belong to the bacillus family. Strains belonging to Bacillus genus are suitable for MICP applications as they can tolerate adverse environmental stresses such as alkaline pH [34].
References
- Alpan I (1970) The geotechnical properties of soils. Earth-Science Reviews 6(1): 5-49.
- Ochsner TE, Horton R, Ren T (2001) A new perspective on soil thermal properties. Soil Science Society America Journal 65(6): 1641-1647.
- Farouki OT (1981) Thermal properties of soils. United States Army Corps of Engineers 151.
- Hamdeh NH, Reeder RC (2000) Soil thermal conductivity: Effects of density, moisture, salt concentration and organic matter. Soil Science Society America Journal 64(4): 1285-1290.
- Wierenga PJ, Nielsen DR, Hagan RM (1969) Thermal properties of soil based upon field and laboratory measurements. Soil Science Society of America Journal 33(3): 354-360.
- Bhojani P, Thakur LS, Shah DL (2021) Study of thermal conductivity of soils for varying density and water content profiles. Springer 133: 143-156.
- Yahaya MY, Nordin N (2010) The development of thermal conductivity measurement apparatus. National Advanced Materials and Manufacturing Engineering Conference (NAMME), Malaysia.
- ASTM, Standard test method for determination of thermal conductivity of soil and soft rock by thermal needle probe procedure, D5334-14.
- ASTM, Standard test method for steady-state heat flux measurements and thermal transmission properties by means of the guarded-hot-plate apparatus, C177-13.
- Xamán J, Lira L, Arce J (2009) Analysis of the temperature distribution in a guarded hot plate apparatus for measuring thermal conductivity. Applied Thermal Engineering 29(4): 617-623.
- Hamuda SS, Rouainia M, Clarke BG (2010) New thermal cell for measuring thermal conductivity of soils. In: Laue, Seward (Eds.), Physical Modelling in Geotechnics, (1st edn), Taylor & Francis Group, pp. 1459-1463.
- Milun S, Kilic T, Bego O (2005) Measurement of soil thermal properties by spherical probe. IEEE Transactions on Instrumentation and Measurement 54(3): 1219-1226.
- Low JE, Loveridge FA, Powrie W, Nicholson D (2014) A comparison of laboratory and in situ methods to determine soil thermal conductivity for energy foundations and other ground heat exchanger applications. Springer 10(2): 209-218.
- DeVries DA (1963) Thermal properties of soils. In: Wijk WR (Eds.), Physics of the Plant Environment. John Wiley & Sons, New York, USA, pp. 210-235.
- Johansen O (1975) Thermal conductivity of soils, Ph.D. thesis, University of Trondheim, Trondheim, Norway. US Army Corps of Engineers, Cold Regions Research and Engineering Laboratory, Hanover, N. H. CRREL Draft English Translation, p. 637.
- Cote J, Konrad JM (2005) A generalized thermal conductivity model for soils and construction materials. Canadian Geotechnical Journal 42(2): 443-458.
- Lu S, Ren TS, Gong Y, Horton R (2007) An improved model for predicting soil thermal conductivity from water content at room temperature. Soil Science Society of American Journal 71(1): 8-14.
- Chen SX (2008) Thermal conductivity of sands. Heat Mass Transfer 44(10): 1241-1246.
- Haigh SK (2012) Thermal conductivity of sands. Geotechnique 62(7): 617-625.
- Likos WJ (2013) Modeling thermal conductivity dryout curves from soil-water characteristic curves. Journal of Geotechnical and Geoenvironmental Engineering 140(5): 04013056.
- Lu N, Dong Y (2015) Closed-form equation for thermal conductivity of unsaturated soils at room temperature. Journal of Geotechnical and Geoenvironmental Engineering 141(6): 04015016.
- Zhang N, Wang ZY (2017) Review of soil thermal conductivity and predictive models. International Journal of Thermal Sciences 117: 172-183.
- Zhang N, Yu XB, Pradhan A, Puppala AJ (2015) Thermal conductivity of quartz sands by thermo-time domain reflectometry probe and model prediction. Journal of Materials in Civil Engineering 27(12): 0401509.
- Paassen LA, Ghose R, Linden T, Star W, Loosdrecht M (2010) Quantifying biomediated ground improvement by ureolysis: Large-scale biogrout experiment. Journal of Geotechnical and Geoenvironmental Engineering 136: 1721-1728.
- Cheng L, Shahin MA, Chu J (2019) Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotechnica 14: 615-626.
- Mujah D, Shahin MA, Cheng L, Karrech A (2021) Experimental and analytical study on geomechanical behavior of biocemented sand. International Journal of Geomechanics 21(8): 04021126.
- Yang Y, Chu J, Cao B, Liu HL, Cheng L (2020) Biocementation of soil using non-sterile enriched urease-producing bacteria from activated sludge. Journal of Cleaner Production 262: 121315.
- Wijngaarden WK, Vermolen FJ, Meurs GA, Vuik C (2011) Modelling biogrout: A new ground improvement method based on microbial-induced carbonate precipitation. Transport in Porous Media 87: 397-420.
- Shahraki RS, O’Kelly BC, Niazi A, Zomorodian SM (2015) Improving sand with microbial-induced carbonate precipitation. Proceedings of the Institution of Civil Engineers Ground Improvement 168(3): 217-230.
- Wang Z, Zhang N, Ding J, Lu C, Jin Y (2018) Experimental study on wind erosion resistance and strength of sands treated with microbial-induced calcium carbonate precipitation. Advances in Materials Science and Engineering 2018: 1-10.
- Paassen LA, Harkes MP, Zwieten GA, Zon WH, Star WR, et al. (2009) Scale up of biogrout: A biological ground reinforcement method. Proceedings of the 17th International Conference on Soil Mechanics and Geotechnical Engineering, Lansdale IOS Press, Alexandria, Egypt, pp. 2328–2333.
- Šovljanski O, Pezo L, Grahovac J, Tomić A, Ranitović A, et al. (2022) Best-performing Bacillus strains for microbiologically induced CaCO3 precipitation: Screening of the relative influence of operational and environmental factors. Journal of Biotechnology 350: 31-41.
- Sohail MG, Disi ZA, Zouari N, Nuaimi NA, Kahraman R, et al. (2022) Bio self-healing concrete using MICP by an indigenous Bacillus cereus strain isolated from Qatari soil. Construction and Building Materials 328: 126943.
- Henze JJ, Randall DG (2018) Microbial induced calcium carbonate precipitation at elevated pH values (>11) using Sporosarcina pasteurii. Journal of Environmental Chemical Engineering 6(4): 5008-5013.
© 2023 Shivani Mistry, This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






