- Submissions

Full Text
Significances of Bioengineering & Biosciences
Application of SERS Nanoparticles for Imaging for Fast, Sensitive and Selective Cancer Screening: A Review
Serana Nelson and Tahrima B Rouf*
Stephenson School of Biomedical Engineering, University of Oklahoma, USA
*Corresponding author:Tahrima B Rouf, Stephenson School of Biomedical Engineering, University of Oklahoma, Norman, Oklahoma-73072, USA
Submission: February 02, 2023 Published: March 30, 2023

ISSN 2637-8078Volume6 Issue1
Abstract
Surface Enhanced Raman Spectroscopy (SERS) is a rapid, label-free, modular, non-destructive, and multiplexed imaging technique used in a number of applications for both elemental and biological analysis. Its flexible, sensitive, and adaptive nature makes SERS an excellent platform for cancer imaging. In the context of nanomedicine, SERS has shown tremendous utility. Several different nanoparticles have been used in SERS for cancer imaging, and includes the most widely studied gold nanoparticles, with silver nanoparticles, carbon-based nanoparticles, and metal oxide semiconductor nanoparticles gaining popularity in recent years. SERS nanoparticles can be tuned to suit nearly any application as the nanoparticle cores, surface coatings, and targeting moieties can be strategically designed for use in biological applications for in situ, in vivo, in vitro, and ex vivo analyses. Since SERS is an emerging cancer diagnostic technology, frequent reviews of the SERS nanoparticle schemes are necessary to inform the research community while ensuring the most cutting-edge SERS technologies continue to be developed. In this review, SERS nanoparticles used in the past five years for cancer imaging will be analyzed, with a focus on the type of nanoparticle as well as the type of cancer to which it is applied.
Keywords: Gold nanoparticles; Silver nanoparticles; Carbon nanoparticles; Ovarian cancer; Breast cancer
Abbreviations:SERS: Surface Enhanced Raman Spectroscopy; NP: Nano Particles; EF: Enhancement Factor
Introduction
Cancer continues to be one of the largest challenges facing modern medicine. A disease characterized by abnormal, uncontrollable cell growth, as well as an extremely heterogeneous microenvironment, there is an urgent need for more precise and accurate techniques for diagnosis, treatment, and monitoring. While there have been several advances in the field of cancer research in the twenty-first century, there are many challenges that remain. Among these challenges, accurate and early diagnosis for the many different types of cancer is currently one of the most urgently studied problems, as it is vital to patient outcomes and survival [1]. Current diagnostic tools and imaging techniques are accurate but suffer from poor sensitivity which hinders early diagnosis [2]. The pathological techniques are costly and time-intensive, thus revealing an urgent need for cancer detection that is a fast, accurate, highly sensitive and selective screening method on the cellular level [1]. Because cancer is a broad disease that possesses a multitude of biomarkers, symptoms, tissue types, and clinical presentation, a multifaceted approach for diagnostics is necessary to ensure accurate and timely diagnosis, treatment, and monitoring. An emerging strategy for the diagnosis and monitoring of cancer is Surface Enhanced Raman Spectroscopy (SERS). SERS is based on a phenomenon called Raman scattering. First discovered by Indian physicist C.V. Raman in 1928, Raman scattering describes the unique interactions of light with matter [3-5]. SERS is an analytical tool with strong multiplexing capabilities that involves amplifying the Raman signal using noble metal-based, metal oxidebased, or hybrid nanoparticles with materials adsorbed to the surface and has been used in materials science, analytical chemistry, trace detections, chemical sensors, food safety, and biochemistry by exploiting the characteristic vibrational fingerprint spectrum and in situ detection analysis [6-12]. SERS offers excellent sensitivity (up to 10–15M), high signal-to-background ratio, specific fingerprint signatures, photostability, and strong multiplexing capabilities [13]. SERS nanoparticles can be customized in a near-infinite number of ways to detect specific particles in a sample. It is this modularity, combined with Raman spectroscopy’s rich structural specificity, experimental adaptability, and extraordinarily high sensitivity made possible by the optical signal’s amplification by precisely adjusted plasmonic nanostructures that makes this method an exciting and powerful tool in cancer diagnostics [1].
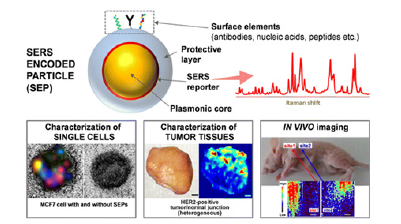
SERS can be used to detect circulating cancer cells, single cancer cells, and tumor microenvironment; it can be used for liquid biopsy as well as multimodal imaging [14,15], cancer cell imaging [16], targeting cancer cells [3,5], 3D cell imaging [5], component analysis [17,18], assisting in tumor resectioning /excision [19], and more recently, theranostics [13]. (Figure 1) depicts an illustrative graphical outline of representative examples of a SERS Encoded Nanoparticles (NP) and its applications in cancer characterization. Current research is focused on addressing the challenges SERS presents that hinder the technique from being clinically integrated. Some challenges include constructing easily reproducible and monodisperse SERS particles that retain their physicochemical properties in biological samples; developing homogenous SERS bioimaging probes that possess excellent spectral reproducibility and sensitivity; constructing bioprobes for cancer cell imaging without the conjugation of a Raman reporter molecule; and finally, the modification of various signal molecules under different laser illuminations in situ to construct multi-label SERS bioimaging probes [20,21]. We are confident that further in-depth research into the use of nanoparticles in SERS in vivo imaging will pave the way for more effective clinical diagnosis and treatment planning for cancer. To further understand SERS nanoparticle compositions and properties, in vivo imaging applications, and the benefits of SERS imaging technology on clinical treatment outcomes, more research on SERS NPs is required. There is a considerable amount of material related to SERS available in the literature. Much of the reviews dedicated to SERS provide a more general outlook of the technology itself as well the design, development, evaluation, and future perspectives of SERS applications related to biotechnology 3/24/2023 10:35:00 AM [22-25]. Some of these reviews have a more specific focus for SERS applications related to cancer diagnosis and imaging [1,26-29]. Other reviews are aimed at the surface modifications, surface coatings, and labels of SERS NPs [20,30,31]. There are a few reviews dedicated to the SERS nanoparticles themselves, however, they are limited to the NP properties and synthesis effects over SERS performance [32]. A recent paper by [33]. reviews SERS nanoparticles in terms of their applications for in vivo imaging different types of cancer [33]. There are no reviews providing a complete, cohesive assessment of all types of SERS NPs used for cancer imaging, diagnostics, and treatment emphasizing their enhancement factors and efficacy. Furthermore, as the field of SERS related to oncology continues to expand exponentially, frequent reviews and updates of the literature are necessary. This review looks at SERS reports from the perspective of the nanoparticle. The different types of nanoparticles used, their construction, and their efficacy in imaging cancer cells were the main focus during organization of previous research. While other reviews are categorizing the application of SERS with respect to different types of cancers, this review is looking at the use and functionality of the different types of surface enhancements/nanoparticles, which can help future SERS researchers in their selection of SERS NPs to achieve specific enhancements for specific types of cancer. This review will pay close attention to the most recent developments in SERS-active nanoparticles, their construction techniques, and targeting molecules to produce SERS-active nanoprobes for the imaging and diagnosis of the most prevalent forms of cancer, with an emphasis on emerging technologies and their applications to specific cancer types for in vivo imaging.
Figure 1:Illustrative depiction of a typical SERS NP along with images of cancer imaging applications with (i) SERS imaging of a single MCF-7 human breast cancer cell; taken from Nima et al. with permission (2014). 2014, Nature Publishing Group, copyright. (ii) Ex vivo SERS imaging of tumor tissue; taken from Wang et al. with permission (2016). 2016 Nature Publishing Group copyright. (iii) In vivo SERS imaging of an intravenously injected tumor at two distinct sites.
Principle of SERS
When light encounters matter, most of the photons are scattered elastically through the Rayleigh effect, however, some of these photons are scattered inelastically [34]. The inelastic scattering of the monochromatic light results in an energy exchange between the photons and scattering material, which causes the photons to be frequency-shifted in a characteristic way [34,35]. Every element, molecule, atom, and material inelastically scatters photons in a way unique to that material. This inelastic scattering is unique to each element or material, just as fingerprints in humans are unique to the individual [35]. Raman spectroscopy studies molecular vibrations by detecting these characteristic frequency shifts to provide molecular information for any sample [35]. The SERS Enhancement Factor (EF) provided by the nanoparticle/substrate can be quantified by the following equation [Eq. 1]
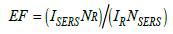
where NSERS and NR stand for the number of molecules evaluated in a Raman and SERS experiment, and ISERS stands for the intensity of the SERS signal and IR for the intensity of the Raman signal [27]. To accurately estimate the SERS enhancement factors, one must know precisely the active surface for the NP or substrate because the quantity of molecules evaluated in a SERS experiment is greatly reliant on the packing density of the analyte molecules adsorbed to the nanoparticle surface [27].
Direct and indirect detection
When performing SERS there are two main modes of Raman signal detection and they are classified by the type of targeting strategy that is used: Direct detection and indirect detection [36]. Figure 2 illustrates these detection strategies while (Figure 3) demonstrates the construction of a typical SERS NP for indirect detection. Direct detection is also known as label-free detection and utilizes “bare” nanoparticles for identifying characteristic SERS spectra for the analyte of interest [27], meaning the SERS spectra that are collected are unique to the target molecule [27]. This is a powerful tool for identifying analytes whose chemical structure is rich in aromatic rings and unsaturated bonds [27]. Direct detection commonly uses metal-based nanoparticles and relies on the high affinity of the target analyte towards the metallic surface, which allows SERS-based detection in complicated, heterogeneous samples [37]. Direct detection is a more straightforward approach but can be difficult to use in biological applications due to the nature of the chemical bonds in biomolecules [38] as well as issues with opsonization that limit signal intensity and selectivity [27]. More recently direct SERS sensing techniques have been developed for nucleic acid detection in biological samples [39], in situ detection of cancer biomarkers [19], cancer characterization through exosome analysis [17], and cancer biomarker quantification in liquid biopsy samples [40].
Figure 2:Schematic comparison of direct vs. indirect detection using plasmonic NP cores (PNPS). Adapted with permission from [36].
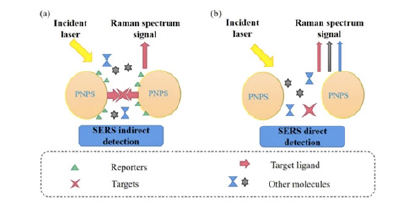
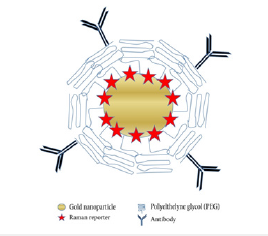
Figure 3:Schematic of NP core, Raman reporter, targeting molecule, and surface coating. Adapted with permission from [32].
The second mode of Raman signal detection is known as indirect detection. Also known as label-based detection, indirect SERS detection relies on SERS nanotags, known as Raman reporters, typically absorbed to noble metals acting as SERS substrates to selectively recognize the target species [25,41]. In this method, a SERS nanotag is needed in addition to a biorecognition molecule like an aptamer or antibody resulting in an extra step in the fabrication process [25,41]. Indirect detection is used in biological samples, whereas direct detection is limited due to the complex nature of a biological environment as well as the chemical bonds in biomolecules. In this detection format, analytes or biomarkers are attracted to a SERS substrate using a molecular recognition moiety, and the successful capture of the analyte or biomarker is then verified by observing the robust SERS signal of the Raman reporter molecule attached to the SERS substrate [42]. Upon recognition of the target analyte, the SERS tag binds to the target molecule to provide both recognition and localization information [27]. The SERS signal recorded belongs to the Raman reporter used, not the target molecule [27]. It is crucial to use reliable targeting molecules while also minimizing nonspecific tag binding, as the reporter molecule’s SERS signal serves as evidence that the target molecule is present [27]. Dyes and thiols are commonly used as Raman reporter molecules and will be discussed in depth in a later passage [30,38]. Indirect SERS detection has been used in a variety of applications such as to create immunoassays [10], SERS biosensors for detection of multiple breast cancer biomarkers in cancer cells [6], sensitive and simultaneous detection of bacterial pathogens [43], and as a precision theranostic platform for in vivo SERS imaging and cancer photothermal therapy [9]. However, in indirect detection, target proteins’ SERS spectra are disregarded, and the reported spectra lack the targets’ detailed structural and chemical information [44]. Each detection strategy offers its own advantages and drawbacks and is dependent on the types of nanoparticles, surface coatings, and Raman reporters used, therefore care must be taken when determining which method to use [27]. In the following passages the main components of SERS labels will be discussed, as well as their effect on SERS performance, while highlighting their applications in cancer imaging and diagnostics.
Main components of SERS labels
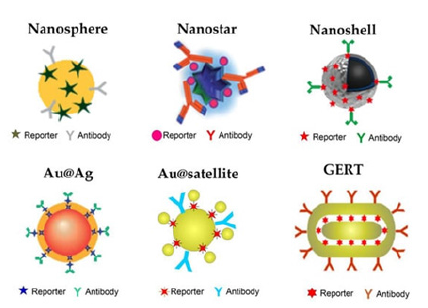
the following components: a plasmonic nanoparticle acting as the core, a Raman reporter molecule, and/or a targeting molecule such as an aptamer or antibody adsorbed to the surface of the core [45]. This section will discuss the rational design of SERS nanoparticles for Raman signal enhancement. Raman scattering intensities can be amplified in several different ways. Understanding the enhancement factors of SERS active substrates is paramount in producing the most effective SERS particles. The EF and EF mechanisms are the key criteria for assessing and manipulating the performance of SERS substates [46,47]. Researchers have finally agreed on the mechanisms governing Raman signal enhancement: Electromagnetic (EM) Enhancement Mechanisms and Chemical (CM) Enhancement Mechanisms [48]. The EM enhancement results From Localized Surface Plasmon Resonance (LSPR), generated from a group oscillation of metallic nanoparticles’ free electrons (NPs) stimulated by a specific frequency of incident light resonating with the incident optical field, enhancing the EM field in the proximity of metal-based NPs [47]. Regions with dramatically amplified EM field intensity are called ‘hot spots’ and will be discussed in more detail in the section pertaining to Raman reporters [47]. EM enhancement can amplify the Raman signal by a factor of 102 to 108 and is the dominating mechanism for the performance of SERS substrates when using metal-based NPs, while the chemical enhancements mechanisms are weak [48,49]. Chemical enhancement mechanisms dominate SERS performance for semiconductor materials like metal oxides and composite-based materials, while their EM enhancement mechanisms are negligible [49]. The CM enhancement of SERS substrates is based on a few different phenomena and arises from the charge transfer of the nanoparticle core and molecules adsorbed to the surface of the NP [47]. Exciton, charge-transfer, and molecular resonances are what contribute to the Raman signal enhancement for semiconductor materials [47]. and can amplify the Raman signal by a factor of 102-103 [48]. NPs fabricated from semiconductor materials demonstrate superior tunable electronic structures when compared to metal-based NPs, as well as a more uniform SERS signals, better biocompatibility, and lower cost [49]. Selection of appropriate materials for fabricating SERS nanoprobes is of the utmost importance to obtain significant Raman signal enhancement. The size and shape of the nanoparticle, followed by the material type and surface characteristics have the greatest effect on the SERS NPs optical properties and targeting abilities [45,47]. Figure 4 illustrates some popular shapes and formulations used for SERS NPs. The core of SERS substrates can be constructed from metals, metal oxides, or composite-based materials [21]. Since the discovery of SERS in 1978, noble metals have been the dominating material of interest for producing SERS-active NPs with gold being the most common and widely investigated, followed by silver, then copper [24]. More recently, metal-oxides and composite materials have proven to be desirable materials for construction of SERS substrates [50].
Figure 4:Schematic illustration of some typical SERS NP shapes and formulations. Adapted with permission from [50].
Raman reporters: For indirect SERS imaging, Raman reporter molecules are used to detect and record the SERS signal within biological samples. The general make-up of a SERS NP with a Raman reporter molecule used for indirect detection is displayed in (Figure 3), while (Figure 5) depicts the general design of a SERS tag used in indirect detection. Raman reporter molecules are commonly referred to as Raman dyes, reporter molecules, and Raman reporters [41,44]. Raman reporters are typically placed within close proximity to the SERS NP to maximize Raman signal enhancement [44]. The Raman dye conjugation is commonly done before adding surface coatings or targeting molecules to the NP surface [44]. Design and selection of appropriate Raman reporters is necessary for significant signal enhancement. Raman reporters possessing resonance with the excitation laser, in addition to a targeting molecule that specifically binds to the biomolecule of interest, are adsorbed to the surface of NPs to achieve the theorized femto-to attomolar limits of detection [30,51]. Necessary features that Raman reporter molecules must display include low sensitivity to laser light, the capacity to bind to the metallic core, strong Raman scattering cross-sections to produce intense signal responses, and Raman spectra with a small number of peaks to reduce peak overlap and increase selectivity, especially in multiplexing experiments [27,31]. It is important to note that ideal Raman reporters should display low water solubility, as reporters with high water solubility can desorb from the NP core because of the complex biological environment, which can compromise the stability and reproducibility of SERS signals from the SERS labels [31]. A desirable feature of Raman reporters is their ability to perform in the Raman silent region, which is a spectral window from 1750-2750cm-1; Raman bands from biological tissues and molecules are minimized in this spectral region [30]. The Raman shift is relative to the energy of the excitation source; therefore, the Raman-silent region corresponds to a wavelength range of roughly 720-775nm when the excitation wavelength is 638-nm and a range of 910-1000nm for a 785nm laser excitation [30]. A Raman reporter molecule with a strong, distinct signal in this region would allow for strong SERS spectra within biological samples [52]. There are many different molecules used as Raman reporters in literature. Statistical and computational models have recently been employed for the rational selection of Raman reporters. This includes schemes related to density functional theory [53], correlation matrices, and the use of linear discriminant analysis [51] to select the most appropriate Raman reporter for specific applications. Raman dyes such as methylene blue, crystal violet, and malachite green are widely used in cell imaging due to their strong signal intensity [51]. Thiolated molecules like 4-methoxythiophenol and 4-mercaptobenzoic acid (4-MBA) are frequently used in indirect detection schemes because of their exceptional ability to conjugate directly to the surface of gold NPs [51]. Other molecules commonly used as Raman reporters include compounds containing amines, like CyNAMLA, a lipoic acidcontaining Near Infrared (NIR) ultrasensitive tricarbocyanine molecule [31,32,47]. NIR active Raman reporter molecules have gained significant interest for improving NIR SERS sensitivity. For in vivo biomedical applications, NIR lasers are preferred due to their extensive penetration depth and minimal autofluorescence/ absorption interference, but their application in SERS imaging has been hindered due to a low SERS signal when NIR lasers are used [31]. The molecule 3,3′-Diethylthiatricarbocyanine (DTTC) is considered the standard for NIR SERS but shows only a moderate Raman intensity [31]. Another way of improving the sensitivity and selectivity of SERS nanoprobes is through surface modifications. The following section will detail the multitude of surface modifications that can be performed, as well as their effects on SERS substrate performance.
Figure 5:Overview for the design of a SERS tag formed by a Raman reporter, plasmonic core, targeting ligands, and colloidal stability coating. Adapted with permission from [52].
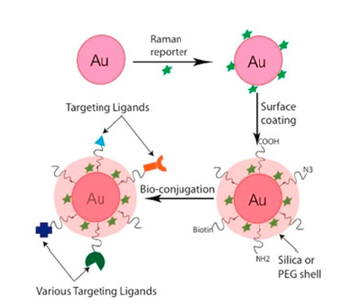
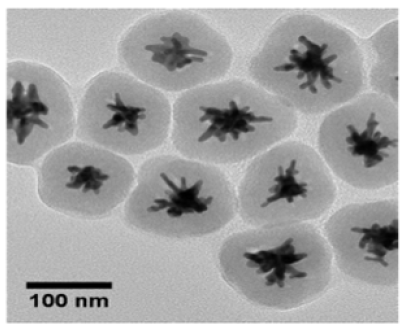
Surface coating: The surface characteristics of SERS NPs are extremely important, as many challenges associated with SERS NP production are related to their colloidal stability, especially in biological environments, leaching of the SERS probe or Raman reporter molecules, and the functionalization and immobilization of molecules at the particle surface [20]. A protective coating on the shell of the NP core can be incorporated to prepare stable and selective SERS labels for in vitro and in vivo applications [31]. Figure 5 provides a general depiction of this scheme. The surface coating improves NP stability and prevents the desorption of the reporter molecules from the particle surface while preserving the intense and distinct Raman fingerprint in the physiological environment [28]. Additionally, the external stabilization coating provides functional groups on the surface for further bioconjugation for selective targeting [20]. This section will summarize the numerous surface modifications that can be made to SERS NPs for specific applications in SERS imaging for cancer diagnostics which include molecules to prevent aggregation as well as functional groups, ligands, aptamers, antibodies, targeting moieties, etc. to selectively target and recognize cancer biomarkers in vitro and in vivo. Poly(Ethylene Glycol) (PEG) is the most widely used polymeric ligand for imparting colloidal stability to nanoparticles in aqueous solutions [20]. The stability and biocompatibility of PEG within biological environments is both well-studied and proven, most notably for its use in the COVID-19 vaccines [54]. PEG is highly hydrophilic in nature which provides this polymer with steric stabilization, causing short-range repulsive hydration around the NP it surrounds, imparting excellent long-term stability in high salt concentrations and high pH, while allowing the NP to avoid unintended protein adsorption [55,56]. Other substances used to impart colloidal stability to SERS NPs include zwitterionic ligands [20]. Zwitterionic molecules are molecules that contain at least two functional groups with mixed charges, resulting in a net charge of zero for the NP [20]. Zwitterionic ligands used as surface coatings have resulted in NPs with smaller hydrodynamic diameter, much lower degrees of opsonization, excellent stealth properties resulting in minimal protein adsorption, low non-specific cellular uptake, and increased blood circulation times compared to PEG-coated SERS NPs [57-63]. Other common surface coatings used for imparting colloidal stability to SERS NPs include Bovine Serum Albumin (BSA) and silica shell coatings [64,65]. Figure 6 details a gold nanostar with a silica coating [66]. The remainder of this paper will detail the specific SERS applications for cancer imaging organized by the types of SERS nanoparticles used followed by the type of cancer with which it is used, beginning with those affecting the female anatomy as they are the most widely investigated. Aside from colloidal stability, many other surface coatings are used with SERS NPs for cancer imaging applications in both direct detection and indirect detection, either for targeting specific cell types/biomarkers or for Raman signal enhancement. For SERS direct detection schemes, ligands or targeting moieties attached to SERS NPs exploit the overabundance of cell surface receptors often overexpressed on cancer cells. Folic acid attached to the SERS NP surface has been frequently used to target folic acid receptors overexpressed on breast, cervical, and ovarian cancer cells [2,3,15,67]. Additionally, sialic acid and glutamic acid have been used as cancer cell targeting ligands in conjunction with folic acid [2,15]. Deoxyribonucleic Acids (DNA), Ribonucleic Acid (RNA), oligonucleotides, and antibodies have also been used as alternative SERS direct detection schemes for targeting specific biomarkers [6,68,69]. Another way to achieve simultaneous and selective SERS signal enhancement for SERS NPs used in indirect detection schemes is through plasmonic hot spot engineering [25]. Raman reporter tags are unable to rely on analyte-induced clustering and signal enhancement (exploited in direct detection), therefore “hot spots” on the NP surface must be engineered [41]. Plasmonic materials strategically positioned on the SERS NP surface can generate significant electromagnetic enhancement of the signal, generated by the surface plasmon effect [70]. When monochromatic laser light illuminates metallic nanostructures, an excitation of the collective oscillations of the surface conduction electrons occurs [25]. In metallic or plasmonic SERS NPs, the surface electrons are subjected to the competing forces from the electric field caused by the monochromatic light as well as the restoring forces caused by the positively charged nuclei, resulting in a simple harmonic motion with an intrinsic resonant frequency known as localized surface plasmon resonance (LSPR) [25]. This leads to easily detectable and localized hot spots on the NP surface responsible for generating the SERS signal. Based on the brief description of some common surface modifications that can be done to SERS NPs, their modularity and customizability are evident. As more SERS NPs with increasing novelty are investigated, their utility in cancer imaging and diagnostics will ensure they remain as powerful tools in nanomedicine.
Figure 6:TEM image of silica-coated gold nanostars used for SERS detection and singlet-oxygen generation as nanotheranostic platform. Adapted with permission from [66].
SERS Nanoparticles for Cancer Imaging
Surface enhanced Raman spectroscopy is emerging as a powerful tool for cancer imaging. While it has not yet been adopted clinically, there are a multitude of studies for cancer imaging using SERS that show great promise. Each cancer type possesses different disease biomarkers, pathology, and clinical presentation, therefore the strategies for imaging and diagnosis are also different. This section will discuss SERS applications for cancer imaging that have emerged within the last five years, with an emphasis on the nanoparticle core, the targeting molecules, type of application, and efficacy of the methods studied. With the information presented, it will be clear that SERS deserves the attention it has garnered and could be the long-awaited answer to the cancer imaging problem that has plagued researchers for a century
Gold nanoparticles for cancer imaging using SERS
Ovarian cancer: The American Cancer Society ranks ovarian cancer as the fifth leading cause of cancer death in women in the United States and for the year 2022 projects roughly 20,000 new cases to be diagnosed in the United States with roughly 13,000 deaths [71]. Ovarian cancer has the highest mortality rate for gynecological malignancies [72,73]. The five-year survival for patients with stage I and II ovarian cancer approaches 95% but is less than 30% for patients diagnosed with stage III and IV, making early diagnosis paramount for patient outcomes [72,73]. Ovarian cancer is considered a silent killer, as it is often asymptomatic in its early stages and left undiagnosed until it has advanced [72]. Ovarian cancer can be highly heterogeneous, with numerous protein markers and cancer-related genes identified as diagnostic, prognostic, and targeted therapy biomarkers in ovarian cancer management [72]. Presently there are no screening strategies recommended for early-stage identification of ovarian cancer[72]. Several research groups have investigated SERS-based cancer imaging strategies that show great promise for early-stage ovarian cancer detection [72,74-76]. The Folate Receptor (FR), which is overexpressed in more than 70% of primary ovarian malignancies and has received substantial research for both targeted therapy and intraoperative imaging, is a prominent biomarker for ovarian cancer [3]. One way to improve survival for ovarian cancer patients is through complete surgical removal of the tumors, however, this is not possible in the early stages of the disease, as surgeons are unable to visualize these microscopic malignancies and 75% of patients already present with tumor metastasis upon initial diagnosis [3]. Researchers from the Memorial Sloan Kettering Cancer Center and the Weill Cornell Medical College have proposed a method to improve tumor identification and resection termed TAS3RS, which is short for topically applied surface enhanced resonance Raman ratiometric spectroscopy [3]. Researchers proposed that local, intraperitoneal administration of FR-targeted SERRS-NPs could detect microscopic ovarian cancer metastases using intravenously administered surface enhanced resonance Raman scattering nanoparticles (SERRS-NPs), which are made up of a gold nanostar core with silica shell. Using mice as animal models and antibody directed FR-targeting alongside ratiometric information with builtin reference standards based on the differential homing of anti-folate receptor SERRS-NPS, researchers were able to detect tumors as small as 370μm, as confirmed with Bioluminescence Imaging (BLI) and histological staining. (Figure 7) offers a detailed comparison of lesions detected with both SERS and BLI with histological staining. The SERS-generated image agrees with the results garnered from imaging and staining. Additionally, the TAS3RS ratiometric algorithm was able to differentiate between the healthy control groups and diseased groups. The four healthy control mice did not display a positive signal, but in the tumor-bearing mice, TAS3RS was able to identify tumorous lesions that had been detected by bioluminescence imaging with great specificity [3]. The intravenous application of this method is limited because the primary route for ovarian cancer metastasis occurs through peritoneal spread rather than through the bloodstream. Although more work must be done before this method can be adopted clinically, it is an encouraging development for the early diagnosis and treatment of ovarian cancer. Gold-based SERS nanoprobes have been studied to efficiently and simultaneously perform spatially resolved in situ measurements for the expression of several ovarian cancer-related markers in tumors [15]. Verdin et al. fabricated gold core silver shell poly(allylamine)-coated nanoparticles as SERS nanoprobes, and the designed SERS nanoprobes were fully characterized by a series of physicochemical techniques. Researchers developed an analytical strategy which enabled them to visualize the spatial localization of the nanoprobes while simultaneously evaluating the relative expression of FRs and Sialic Acid (SA) of cancerous tissues in comparison to normal tissues [15]. They demonstrated the targeting capability of the nanoprobes while confirming the overexpression of FR and SA on cancer cells [15]. The high accuracy of the nanoprobes’ ability to spatially resolve the tumor was confirmed with histology and suggests this strategy provides even greater molecular information than histology alone [15]. Recently, human epididymis protein 4 (HE4), a serological protein, has been identified as an important clinical biomarker for endothelial ovarian cancer [77]. Gold nanoplate-based SERS immunoassay has been developed to quantitatively detect HE4 in serum samples in quantities as low as 10-17M [75]. In this immunoassay a capture antibody-immobilized gold nanoplate acts as an immune substrate, while detection antibody-immobilized gold NPs (AuNPs) serve as immunoprobes [75]. When HE4 is present in a sample the gold nanoplates and AuNPs form a sandwich structure which generates a strong SERS signal that is easily detectable with Raman microscopy [75]. These results could aid in early ovarian cancer detection and can be expanded to detect numerous other biomarkers.
Figure 7:Comparison between TAS3RS imaging accuracy and BLI and histological results. (A) Upper abdomen multiorgan specimen. (B) BLI findings. (C) The associated TAS3RS map. (D) H&E slides with a 250m interslice gap that were obtained for lesion marking. In both the TAS3RS map and the histology slides, lesions at the same anatomical site are given the same numerical annotation. Arrows in black and white 1-3: Between the hepatic hilum and the lesser curvature of the stomach, metastatic implants. Large infra-gastric implant in pancreatic tissue, shown by arrow 4. Yellow arrow: Histology missed a single perisplenic lesion found by BLI and Raman.Adapted with permission from [3].

Breast cancer: Due to the modular nature of SERS and SERSencoded particles, this platform is excellent for use in multimodal imaging. A 2017 study by [78]. uses silica-coated gold nanoparticles with Raman reporters adsorbed to the surface to perform indirect SERS for ex vivo analysis of breast tissues [78]. SERS NPs were topically applied to excised tissues to rapidly visualize a multiplex panel of cell surface markers present at surgical margins. The platform was able to simultaneously detect and quantify the expression of four different breast cancer biomarkers, ER, HER22, CD44, and EGFR in tissue samples with 89.3% sensitivity and 92.1% specificity [78]. This scheme proves the ability of SERS to rapidly detect biomarkers in freshly excised tissues to assist in tumor excision, intraoperative guidance, and ultimately, cancer diagnosis and treatment. PEGylated silver encapsulated gold hollow nanospheres has been used as SERS nanotags for the identification of localized distribution of numerous protein biomarkers expressed on breast cancer cells [14]. Sk-Br3 and MDA-MB-231 breast cancer cell lines were used as the model systems for the study. The localized distribution of three cancer biomarkers (antiepithelial cell adhesion molecule (EpCAM), anti-erythroblastic oncogene B2 (ErbB2), and anti-cluster of differentiation (CD44)) co-expressed on the breast cancer cells were examined [75]. SERSmapping technology made it simple to identify the detailed local distributions of numerous biomarkers expressed on specific cancer cells when compared to Western Blot data from the various cancer cell lines. Additionally, each biomarker expressed on cells could be quantified by examining corresponding Raman intensities [75]. SERS-active silver@gold NPs coated with Polyethylene Glycol (PEG) were functionalized with monoclonal antibodies which allowed highly specific targeting of HER2 overexpressed on breast cancer cells [5]. Researchers demonstrated the proportional correlation between the SERS signal and the amount of HER2 antigen present on cell membranes in what the authors assert is the very first demonstration of quantitative SERS imaging on tissues using targeting SERS probes [5]. This study also demonstrated 3D imaging of cancer cells as it provided spatially resolved determination of the amount of HER2 presented on breast cancer cells [5].
Lung cancer:Raman reporter-conjugated silver wrapped gold nanorods functionalized with DNA probes for the detection of lung cancer-related microRNAs, miRNA-21, in complex plasma samples have been studied [79]. Through a hybridization-based recognition effect, a large SERS signal enhancement caused by miRNA-21-triggered assembly of core-satellite nanocomposites was observed. The nanocomposites enabled the sensitive detection of miRNA-21 in quantities as low as 0.1fM in a linear range of 10fM to 1nM. Detection of miRNAs is historically difficult, however, this platform provides bio-interference-free, quantitative, and direct SERS imaging of cancer without RNA pre-extraction, thereby demonstrating clinical potential for the selective, sensitive, and accurate quantification and detection of miRNA markers in liquid biopsy samples [79].
Bladder cancer:Gold triangular nanoprisms (AuTNPs) have been synthesized to offer a novel, solid-state nanoplasmonic sensor that can detect circulating miRNAs for the early detection of bladder cancer in liquid biopsy samples [40]. The unique LSPR properties of the AuTNPs’ provided large SERS enhancements as well as plasmon-enhanced fluorescence enhancements. The designed sensors were capable of quantifying bladder cancerrelated miRNAs, miRNA-10b and miRNA-96, in concentrations as low as 0.3fg/μL directly from the plasma of bladder cancer patients. This platform offers an alternative to the clinical “gold standard” of miRNA assays like quantitative reverse-transcription polymerase chain reaction-based technologies (qRT-PCR), while avoiding many of the associated drawbacks which include required labeling and amplification of the sample prior to analysis, complicated laborintensive RNA extraction, complementary DNA conversion steps, and the enormous sample volumes needed for analysis. This analytical method mitigated both false negative and false positive responses while demonstrating the excellent stability of the sensors when exposed to biological liquids. This AuTNP-based SERS cancer detection scheme could eventually be adopted for clinical point-ofcare applications for diagnosing cancer as well as other diseases like SARS-CoV-2, where RNAs are used as biomarkers [40].
Silver nanoparticles for cancer imaging using SERS
Colon and colorectal cancer:Two different types of nanoparticles have been studied to obtain Fluorescence and Surface- Enhanced Raman Scattering (F-SERS) dots for simultaneous use in a Fluorescence-Raman Endoscopic System (FRES) to demonstrate their utility in cancer imaging and diagnosis in an orthotopically induced colorectal cancer xenograft model [80]. The F-SERS dots, composed of both silver NPs and silica NPs, were conjugated with antibodies to specifically target Epidermal Growth Factor Receptors (EGFR) and Vascular Endothelial Growth Factors (VEGF), as proteins related to these signaling cascades serve as predictive biomarkers for colorectal cancer [81]. Two different types of NPs were used to simultaneously emit SERS and fluorescent signals from a singular NP: a fluorescent silica NP shell coating and a Raman active chemically labeled silver NP [82]. In this scheme the silver NP served as the Raman active probe. Researchers successfully demonstrated that the NPs used for FRES can be easily applied to endoscopy and used in multiplex molecular diagnosis; the numerous molecular characteristics of a tumor could be acquired simultaneously when performing a colonoscopy, thereby assisting in cancer diagnosis while providing insight for the most effective treatment options [82].
Glioblastomas:For glioblastoma, the five-year survival rate for patients is only 6.8% and the average length of survival after the initial diagnosis is only eight months; the survival and mortality statistics for glioblastomas have remained virtually unchanged for decades (“About Glioblastoma,” n.d.). A rapid and facile screening method has been tested using silver nanoparticle-decorated silver nanorods (AgNPs@AgNR) with SERS to achieve discrimination between healthy brain tissue and gliomas [83]. The prepared AgNP@AgNR substrates showed excellent SERS performance with an EF up to 1.37x109. The substrates were used in combination with principal component analysis and the SERS spectra obtained from sample tissues demonstrated the platform’s ability to rapidly detect cancerous tissues within samples, providing a basis for a point-ofcare diagnostic approach for glioblastoma.
Multiplex cancer detection:Metastatic cancers involve more than one cell type and often involve multiple forms of cancer; therefore, it is desirable to develop SERS probes capable of detecting multiple malignancies simultaneously [35]. used silver nanoparticles for SERS-based differential diagnosis from serum samples (n=253) representing healthy patients and five solid malignancies: lung, colorectal, ovarian, oral, and breast cancer [35]. Principal component analysis-linear discriminant analysis (PCALDA) was used to evaluate the SERS NPs’ classification accuracy. The specificity and sensitivity of the NPs for discriminating between healthy control samples and cancer patients was 91% and 98% respectively; cancer samples were correctly classified by cancer type with an accuracy of 86% for colorectal cancer, 88% for oral cancer, 80% for ovarian cancer, 59% for lung cancer, and 76% for breast cancer [35]. These results validate the efficacy of SERS for the simultaneous multiplexed diagnosis of several malignancies. More recently, 3-D stacked silver nanowires (AgNWs) have been assembled on a glass fiber filter sensor as a rapid urine analysis system for diagnosing both pancreatic and prostate cancer [84]. The designed sensor was able to discriminate between the three experimental groups which included the prostate cancer group, pancreatic cancer group, and a normal control group [84]. The baseline-corrected and averaged SERS spectra from the prostate cancer and pancreatic cancer groups showed significant SERS enhancement of peaks associated with malignant biomarkers compared to that of the normal control group [84]. The SERS spectral patterns obtained from the urine samples on the AgNW sensor were analyzed by PCA and orthogonal partial least-squares discriminant analysis (OPLS-DA) methods [84]. The AgNW sensor had a specificity and sensitivity of 100% when discriminating between the prostate cancer group and pancreatic cancer group as well as a 100% sensitivity and 100% specificity when discriminating between the combined cancer groups and the healthy control group [84]. These results show that the developed SERS sensor can be used for the noninvasive classification and diagnosis of cancer which is especially encouraging for pancreatic cancer, as this type of cancer possesses a high mortality rate and zero established methods for early diagnosis [84].
Metal oxide nanoparticles for cancer imaging using SERS
Noble metals have remained the focus for SERS substrates and NPs in cancer imaging, however, more recently metal oxides have been gaining momentum in the field. Metal oxides demonstrate good stability, biocompatibility, and sensitivity, making metal oxides and semiconductors excellent candidates for use as SERS substrates and nanoparticles [85]. Metal oxides possess extremely tunable properties including their band gap energies, morphology, size dependent plasmon/exciton resonances, and charge transfers, while their SERS enhancement is dominated by CM enhancement mechanisms [85]. The use of metal oxides as SERS NPs for cancer imaging is described in the following passages.
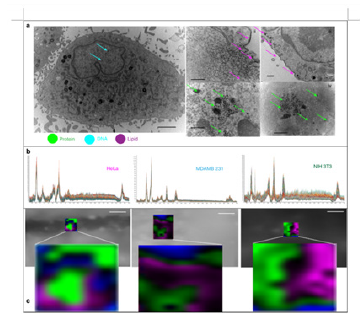
Breast cancer:In a study by [16]., researchers used zinc oxide SERS nanoprobes decorated on a nanodendrite platform to image single breast cancer cells [16]. The quantum probes combined with PCA and DA were able to discriminate between non-cancerous and cancerous cells while simultaneously sensing the RNA, DNA lipids, and proteins in vitro with excellent sensitivity. The designed probes showed SERS EF up to 106 with a detection limit up to the singlecell- level. The SERS spectra obtained from the probes was used to carry out single-cell Raman mapping, with cellular components accurately labeled. Figure 8 shows the single-cell Raman maps obtained with the probes [86]. describe the construction, characterization, and evaluation of crystal-amorphous core-shell structured black titanium dioxide nanoparticles (B-TiO2) modified with a Raman reporter molecule and a targeting antibody applied to MCF-7 breast cancer cells [86]. The synergistic effects from the crystal-amorphous core-shell structure demonstrated remarkable SERS activity with EF up to~105 for the Raman reporter molecule 4NBT and facilitated photo-induced charge-transfer between the target molecules and SERS substrate. These results suggest that B-TiO2 could be used as sensitive and biocompatible SERS probes to identify MCF-7 breast cancer cells quickly and accurately [16]
Figure 8:Single-cell level in vitro detection using SERS. a Simultaneous signal enhancement generated from numerous biomolecules caused by cellular uptake and internalization of probes. (i) Scale bar=2μm (ii-v) Scale bar=500nm. b SERS spectra from single cells confirm that the entrapment of the probes within the lysosomes does not negatively affect signal enhancement. c Single-cell Raman mapping indicating ability of the probe for single-celllevel detection. Scale bar=20μm. Adapted with permission from [16].ss
Prostate cancer:Silica is commonly used as a coating for SERS nanoparticles however, it is also used as the nanoparticle core in SERS applications for cancer imaging [87]. Silica nanoparticles decorated on silver nano-islands have been used as a SERS substrate for the facile and efficient sensing of the prostate cancer biomarker sarcosine [87]. The designed SERS platform had a remarkably low Limit of Detection (LOD) of 1.76nM and an enhancement fact of 2x107. The enhanced Raman signal is attributed to the coupling of several interactions including Whispering Gallery Modes, resonance modes, and the LSPR generated in the silver islands by photonic nanojets in the silica nanospheres. These results are encouraging and provide a promising platform for developing protocols for the early detection of prostate cancer.
Carbon nanoparticles for cancer imaging using SERS
Since the discovery of carbon nanotubes in 1991, followed by the discovery of graphene in 2004, carbon-based nanoparticles have garnered significant attention in the field of nanomedicine [88]. More recently these materials are being explored for their use in SERS for cancer imaging. This includes materials like onedimensional carbon nanotubes, zero-dimensional carbon-based quantum dots, three-dimensional spatial carbon nanomaterials or carbon-based core-shell nanostructures, and two-dimensional graphene and graphene oxide [88]. The use of carbon nanomaterials as SERS NPs for cancer imaging is described in the following passages.
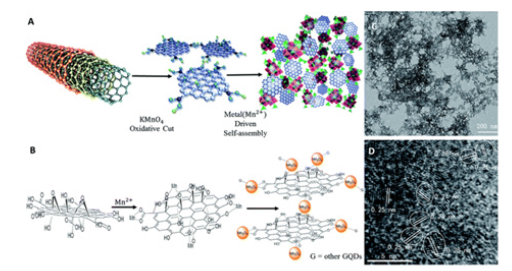
Cervical cancer:Biocompatible self-assembled nanoporous graphene quantum dot-manganese oxide (GQD-Mn3O4) nanocomposites have been studied for SERS-based identification of cancer [89]. The nanocomposites as well as their synthesis strategy are depicted in Figure 9. A one-pot synthesis formed the nanocomposites through self-assembly from multiwalled carbon nanotube (MWCNTs) precursors, resulting in changes to the particles’ energy levels and bandgaps which resulted in a SERS EF of 2.06x104 for the nanocomposites. The GQD-Mn3O4 nanocomposites were able to discriminate cervical cancer cells (HeLa) and liver cancer cells (HepG-2) from healthy cells (7702) providing a cancer imaging scheme for developing new and improved biomedical and bioanalytical SERS applications for cancer imaging [89]. SERS-activated Graphene Oxide (GO) quantum cytosensor in combination with machine learning has been reported for whole cell cancer detection that is capable of screening down to the single-cell level [90]. The quantum cytosensor was synthesized through the multiphoton femtosecond ionization of graphite and its efficacy for cancer screening was tested on three different cell lines: cervical cancer cells (HeLa), breast cancer cells (MDA-MB-231), and healthy fibroblast cells (NIH3T3) [90]. Reducing the size of the cytosensor along with increasing the density of functional group moieties on the sensor surface enabled researchers to gain insight into intracellular molecular processes by monitoring DNA fragmentation, protein denaturation, and protein degradation which are classical markers for cellular apoptosis as well as aiding in the intracellular distribution of the probe. The quantum probes provided increases in SERS enhancement in quantities of 3000-,2500-,3500-fold of DNA, RNA, and protein, respectively. By employing machine learning techniques, discernment of SERS spectral signatures between cancerous and non-cancerous cells was achieved with a high diagnostic specificity and sensitivity of 84.83% and an exceptional accuracy of 92.3% [90]. This research provides a powerful and novel platform for the early screening and diagnosis of cancer.
Figure9:(A) GQD-Mn3O4 nanocomposite synthesis with MWCNTs as the precursor for GQDs. (B) The GQD-Mn3O4 nanocomposite formation mechanism. (C) TEM image of the synthesized nanocomposite. (D) HRTEM image of the nanocomposite. Adapted with permission from [89].
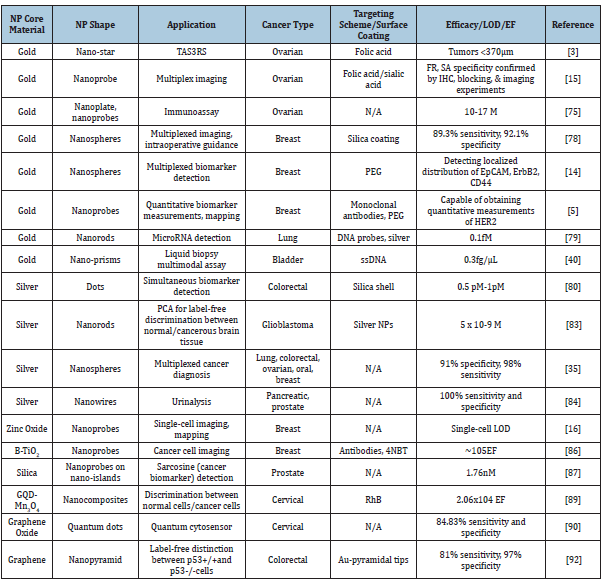
Colon and colorectal cancer:Colorectal cancer is the third most common cancer diagnosed in the United States, with increasing incidences occurring in people under the age of 50 [91]. Diagnosis of colorectal cancer has improved over the years, however, a more complete model is needed as the traditional screening method of colonoscopy may result in misdiagnosis for up to 25% of cases [82]. SERS could offer a new and more efficient diagnosis strategy for colorectal cancers. In 2018, researchers from the University of California proposed a graphene-based SERS platform capable of distinguishing between a TP53 gene knockout cell line (p53-/-) and a wild type cell line (p53+/+) [92]. Pyramidal gold tips decorated on a graphene monolayer combined with Principal Component Analysis (PCA) provided a non-destructive and label-free platform to detect colorectal cancer cells based on the cell surface proteome from a singular gene difference. Raman peak matching combined with PCA provided SERS identification of colon cancer cells with an average 97% specificity and 81% sensitivity [92]. The findings of this research as well as other research that have used nanoparticles for SERS imaging of cancer cells have been summarized in (Table 1).
Table 1:Summary of SERS nanoparticles used for different types of cancer imaging.
Conclusion and Outlooks
The field of nanomedicine is ever evolving, with new nanoparticles and imaging platforms being developed frequently. Surface enhanced Raman spectroscopy is a modular, effective, and powerful imaging technique that uses a wide array of nanoparticles, whose formulations and applications are limited only by the imagination of the researcher. Surface enhanced Raman spectroscopy continues to gain interest in its applications for the diagnosis of cancer. There are numerous applications of SERS for cancer diagnosis, with each showing great promise and efficacy [93]. Although it has not yet been applied in clinical settings, the body of research pertaining to SERS cancer imaging continues to expand and move towards human trials. The challenges preventing SERS from being adopted clinically are being addressed across multiple platforms and research teams, and include constructing easily reproducible and monodisperse SERS particles; developing homogenous material-based SERS bioimaging probes with good spectral reproducibility and sensitivity simultaneously; constructing bioprobes that can be used for cancer cell imaging without the conjugation of a Raman reporter molecule; and finally, the modification of various signal molecules under different laser illuminations in situ to construct multi-label SERS bioimaging probes. Because SERS offers unmatched and label-free sensitivity, selectivity, specificity, and multiplexing capabilities, addressing the aforementioned challenges will advance cancer diagnostics to unprecedented levels, making it distinctly possible to completely eradicate early cancer deaths across the globe
Acknowledgement
The authors would like to thank the National Institute of Health-Centers of Biomedical Engineering Excellence (NIH-COBRE) for their support. This work was supported in part by COBRE grant P20GM135009.
References
- Guerrini L, Pazos PN, Garcia RE, Alvarez PR (2017) Cancer characterization and diagnosis with SERS-encoded particles. Cancer Nano 8: 5.
- Ruiyi L, Tinling P, Hongxia C, Jinsong S, Zaijun L (2020) Electrochemical detection of cancer cells in human blood using folic acid and glutamic acid-functionalized graphene quantum dot-palladium@gold as redox probe with excellent electrocatalytic activity and target recognition. Sensors and Actuators B: Chemical 309.
- Oseledchyk A, Andreou C, Wall MA, Kircher MF (2017) Folate-targeted surface-enhanced resonance raman scattering nanoprobe ratiometry for detection of microscopic ovarian cancer. ACS Nano 11(2): 1488-1497.
- Raman CV, Krishnan KS (1928) A new type of secondary radiation. Nature 121: 501-502.
- Verdin A, Malherbe C, Eppe G (2021) Spatially resolved determination of the abundance of the HER2 marker in microscopic breast tumors using targeted SERS imaging. Microchim Acta 188: 288.
- Huang X, El-SIH, Qian W, El-SMA (2007) Cancer cells assemble and align gold nanorods conjugated to antibodies to produce highly enhanced, sharp, and polarized surface raman spectra: A potential cancer diagnostic marker. Nano Lett 7: 1591-1597.
- Ikeda K, Suzuki S, Uosaki K (2011) Crystal face dependent chemical effects in surface-enhanced raman scattering at atomically defined gold facets. Nano Lett 11(4): 1716-1722.
- Li JF, Huang YF, Ding Y, Yang ZL, Li SB, et al. (2010) Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464: 392-395.
- Lv R, Santos MCD, Antonelli C, Feng S, Fujisawa K, et al. (2014) Large-area Si-doped graphene: Controllable synthesis and enhanced molecular sensing. Adv Mater 26(45): 7593-7599.
- Muehlethaler C, Considine CR, Menon V, Lin WC, Lee YH, et al. (2016) Ultrahigh raman enhancement on monolayer MoS2. ACS Photonics 3(7): 1164-1169.
- Nie S, Emory SR (1997) Probing single molecules and single nanoparticles by surface-enhanced raman scattering. Science 275(5303): 1102-1106.
- Zhu Z, Meng H, Liu W, Liu X, Gong J, et al. (2011) Superstructures and SERS properties of gold nanocrystals with different shapes. Angew Chem Int Ed 50(7): 1593-1596.
- Wang J, Liang D, Jin Q, Feng J, Tang X (2020) Bioorthogonal SERS nanotags as a precision theranostic platform for in vivo sers imaging and cancer photothermal therapy. Bioconjugate Chem 31(2): 182-193.
- Choi N, Dang H, Das A, Sim MS, Chung IY, et al. (2020) SERS biosensors for ultrasensitive detection of multiple biomarkers expressed in cancer cells. Biosensors and Bioelectronics 164.
- Verdin A, Malherbe C, Müller WH, Bertrand V, Eppe G (2020) Multiplex micro-SERS imaging of cancer-related markers in cells and tissues using poly(allylamine)-coated Au@Ag nanoprobes. Anal Bioanal Chem 412: 7739-7755.
- Haldavnekar R, Venkatakrishnan K, Tan B (2018) Non plasmonic semiconductor quantum SERS probe as a pathway for in vitro cancer detection. Nat Commun 9: 3065.
- Carmicheal J, Hayashi C, Huang X, Liu L, Lu Yao, et al. (2019) Label-free characterization of exosome via surface enhanced Raman spectroscopy for the early detection of pancreatic cancer. Nanomedicine Nanotechnology Biology and Medicine 16: 88-96.
- Chen H, Li X, Broderick N, Liu Y, Zhou Y, et al. (2018) Identification and characterization of bladder cancer by low-resolution fiber-optic Raman spectroscopy. J Biophotonics 11(9).
- Desroches J, Jermyn M, Pinto M, Picot F, Tremblay MA, et al. (2018) A new method using Raman spectroscopy for in vivo targeted brain cancer tissue biopsy. Sci Rep 8: 1792.
- Guerrini L, Alvarez-PRA, Pazos PN (2018) Surface modifications of nanoparticles for stability in biological fluids. Materials 11(7): 1154.
- Lin J, Akakuru OU, Wu A (2021) Advances in surface‐enhanced Raman scattering bioprobes for cancer imaging. VIEW 2:(5).
- Langer J, Aizpurua JAD, Javier A, Alvarez-PRA, Auguié B, et al. (2020) Present and future of surface-enhanced raman scattering. ACS Nano 14(1): 28-117.
- Lin S, Cheng Z, Li Q, Wang R, Yu F (2021) Toward sensitive and reliable surface-enhanced raman scattering imaging: From rational design to biomedical applications. ACS Sens 6(11): 3912- 3932.
- Pérez-JAI, Lyu D, Lu Z, Liu G, Ren B (2020) Surface-enhanced Raman spectroscopy: Benefits, trade-offs and future developments. Chem Sci 18: 4563-4577.
- Tahir MA, Dina NE, Cheng H, Valev VK, Zhang L (2021) Surface-enhanced Raman spectroscopy for bioanalysis and diagnosis. Nanoscale 27: 11593-11634.
- Auner GW, Koya SK, Huang C, Broadbent B, Trexler M, et al. (2018) Applications of raman spectroscopy in cancer diagnosis. Cancer Metastasis Rev 37: 691-717.
- Fabris L (2016) SERS tags: The next promising tool for personalized cancer detection? ChemNanoMat 2(4): 249-258.
- Kenry, Nicolson F, Clark L, Panikkanvalappil SR, Andreiuk B, et al. (2022) Advances in surface enhanced raman spectroscopy for in vivo imaging in oncology. Nanotheranostics 6(1): 31-49.
- Liu K, Zhao Q, Li B, Zhao X (2022) Raman spectroscopy: A novel technology for gastric cancer diagnosis. Front Bioeng Biotechnol 10.
- Plakas K, Rosch LE, Clark MD, Adbul-RS, Shaffer TM et al. (2022) Design and evaluation of raman reporters for the raman-silent region. Nanotheranostics 6(1): 1-9.
- Shan B, Pu Y, Chen Y, Liao M, Li M (2018) Novel SERS labels: Rational design, functional integration and biomedical applications. Coordination Chemistry Reviews 371: 11-37.
- Israelsen ND, Hanson C, Vargis E (2015) Nanoparticle properties and synthesis effects on surface-enhanced raman scattering enhancement factor: An introduction. The Scientific World Journal pp.1-12.
- Du Z, Qi Y, He J, Zhong D, Zhou M (2021) Recent advances in applications of nanoparticles in SERS in vivo WIREs Nanomed Nanobiotechnol 13(2).
- Li Y, Wei Q, Ma F, Li X, Liu F, et al. (2018) Surface-enhanced Raman nanoparticles for tumor theranostics applications. Acta Pharmaceutica Sinica B 8(3): 349-359.
- Moisoiu V, Stefancu A, Gulei D, Boitor R, Magdo L, et al. (2019) SERS-based differential diagnosis between multiple solid malignancies: breast, colorectal, lung, ovarian and oral cancer. IJN 14: 6165-6178.
- Li L, Cao X, Zhang T, Wu Q, Xiang P, et al. (2022) Recent developments in surface-enhanced Raman spectroscopy and its application in food analysis: Alcoholic beverages as an example. Foods 11(14): 2165.
- Jahn IJ, Mühlig A, Cialla-MD (2020) Application of molecular SERS nanosensors: Where we stand and where we are headed towards? Anal Bioanal Chem 412: 5999-6007.
- Pilot R, Signorini R, Durante C, Orian L, Bhamidipati M, et al. (2019) A review on surface-enhanced Raman scattering. Biosensors 9(2): 57.
- Garcia-RE, Alvarez-PR.A, Guerrini L (2018) Direct surface-enhanced Raman scattering (SERS) spectroscopy of nucleic acids: From fundamental studies to real-life applications. Chem Soc Rev 47: 4909-4923.
- Masterson AN, Liyanage T, Berman C, Kaimakliotis H, Johnson M, et al. (2020) A novel liquid biopsy-based approach for highly specific cancer diagnostics: mitigating false responses in assaying patient plasma-derived circulating microRNAs through combined SERS and plasmon-enhanced fluorescence analyses. Analyst 145: 4173-4180.
- Fabris L (2015) Gold-based SERS tags for biomedical imaging. J Opt 17.
- Hamm L, Gee A, Indrasekara ASDS (2019) Recent advancement in the surface-enhanced raman spectroscopy-based biosensors for infectious disease diagnosis. Applied Sciences 9: 1448.
- Li Y, Lu C, Zhou S, Fauconnier ML, Gao, et al. (2020) Sensitive and simultaneous detection of different pathogens by surface-enhanced Raman scattering based on aptamer and Raman reporter co-mediated gold tags. Sensors and Actuators B: Chemical 317.
- Ryu HJ, Lee WK, Kim YH, Lee JS (2021) Interfacial interactions of SERS-active noble metal nanostructures with functional ligands for diagnostic analysis of protein cancer markers. Microchim Acta 188: 164.
- Bhamidipati M, Lee G, Kim I, Fabris L (2018) SERS-Based Quantification of PSMA in tissue microarrays allows effective stratification of patients with prostate cancer. ACS Omega 3(12): 16784-16794.
- Cara E, Mandrile L, Sacco A, Giovannozzi AM, Rossi AM, et al. (2020) Towards a traceable enhancement factor in surface-enhanced Raman spectroscopy. J Mater Chem C 8: 16513-16519
- Li M, Qiu Y, Fan C, Cui K, Zhang Y, et al. (2018) Design of SERS nanoprobes for Raman imaging: Materials, critical factors and architectures. Acta Pharmaceutica Sinica B 8(3): 381-389.
- Heeg S, Mueller NS, Wasserroth S, Kusch P, Reich S (2021) Experimental tests of surface‐enhanced Raman scattering: Moving beyond the electromagnetic enhancement theory. J Raman Spectrosc 52(2): 310-322.
- Wang H, Liu Y, Rao G, Wang Y, Du X, et al. (2021) Coupling enhancement mechanisms, materials, and strategies for surface-enhanced Raman scattering devices. Analyst 146: 5008-5032.
- Khlebtsov B, Khlebtsov N (2020) Surface-enhanced raman scattering-based lateral-flow immunoassay. Nanomaterials 10(11): 2228.
- Sánchez-PM, Roig SB, Rodriguez QC, Leonardo BM, Hamad SK (2018) Reporter selection for nanotags in multiplexed surface enhanced raman spectroscopy assays. ACS Omega 3(9): 10733-10742.
- Lane LA, Qian X, Nie S (2015) SERS nanoparticles in medicine: from label-free detection to spectroscopic tagging. Chem Rev 115(19): 10489-10529.
- Mu X, Guo Y, Li Yulong, Wang Z, Li Yuee, et al. (2017) Analysis and design of resonance Raman reporter molecules by density functional theory. J Raman Spectrosc 48(9): 1196-1200.
- Constantin C, Pisani A, Bardi G, Neagu M (2021) Nano-carriers of COVID-19 vaccines: The main pillars of efficacy. Nanomedicine 16(26): 2377-2387.
- Kim J, Kim HS, Lee N, Kim T, Kim H, et al. (2008) Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew Chem Int Ed Engl 47(44): 8438-8441
- Sakura T, Takahashi T, Kataoka K, Nagasaki Y (2005) One-pot preparation of mono-dispersed and physiologically stabilized gold colloid. Colloid Polym Sci 284: 97-101.
- Arvizo RR, Miranda OR, Moyano DF, Walden CA, Giri K, et al. (2011) Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. Plos one.
- Breus VV, Heyes CD, Tron K, Nienhaus GU (2009) Zwitterionic biocompatible quantum dots for wide pH stability and weak nonspecific binding to cells. ACS Nano 3(9): 2573-2580.
- Gupta A, Moyano DF, Parnsubsakul A, Papadopoulos A, Wang LS, et al. (2016) Ultrastable and biofunctionalizable gold nanoparticles. ACS Appl Mater Interfaces 8(22): 14096-14101.
- Longmire M, Choyke PL, Kobayashi H (2008) Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 3(5): 703-717.
- Moyano DF, Saha K, Prakash G, Yan B, Kong H, et al. (2014) Fabrication of Corona-Free Nanoparticles with Tunable Hydrophobicity. ACS Nano 8(7): 6748-6755.
- Muro E, Pons T, Lequeux N, Fragola A, Sanson N et al. (2010) Small and stable sulfobetaine zwitterionic quantum dots for functional live-cell imaging. J Am Chem Soc 132(13): 4556-4557.
- Pelaz B, Alexiou C, Alvarez-PRA, Alves F, Andrews AM, et al. (2017) Diverse applications of nanomedicine. ACS Nano 11(3): 2313-2381.
- Lu L, Yu J, Liu X, Yang X, Zhou Z, et al. (2019) Rapid, quantitative and ultra-sensitive detection of cancer biomarker by a SERRS-based lateral flow immunoassay using bovine serum albumin coated Au nanorods. RSC Adv. 10(1): 271-281.
- Żygieło M, Piotrowski P, Witkowski M, Cichowicz G, Szczytko J, et al. (2021) Reduced self-aggregation and improved stability of silica-coated Fe3O4/Ag SERS-active nanotags functionalized with 2-mercaptoethanesulfonate. Front Chem 9: p. 697595.
- Fales AM, Yuan H, Vo DT, et al. (2011) Silica-coated gold nanostars for combined surface-enhanced raman scattering (SERS) detection and singlet-oxygen generation: A potential nanoplatform for theranostics. Langmuir 27(19): 12186-12190.
- Fasolato C, Giantulli S, Silvestri I, Mazzarda F, Toumia Y et al. (2016) Folate-based single cell screening using surface enhanced Raman microimaging. Nanoscale 8(39): 17304-17313.
- Lee T, Mohammadniaei M, Zhang H, Yoon J, Choi HK, et al. (2020) Single functionalized pRNA/gold nanoparticle for ultrasensitive microRNA detection using electrochemical surface-enhanced raman spectroscopy. Advanced Science 7(3).
- Liu J, Zheng T, Tian Y (2019) Functionalized h‐BN nanosheets as a theranostic platform for SERS real‐time monitoring of microRNA and photodynamic therapy. Angew Chem 131(23): 7839-7843
- Radziuk D, Moehwald H (2015) Prospects for plasmonic hot spots in single molecule SERS towards the chemical imaging of live cells. Phys Chem Chem Phys 17(33): 21072-21093.
- Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA A Cancer J Clinicians 72(1): 7-33.
- Atallah GA, Aziz NHA, Teik CK, Shafiee MN, Kampan NC (2021) New predictive biomarkers for ovarian cancer. Diagnostics 11(3): p. 465.
- Kozak KR, Amneus MW, Pusey SM, Farias ER (2003) Identification of biomarkers for ovarian cancer using strong anion-exchange ProteinChips: Potential use in diagnosis and prognosis. Proc Natl Acad Sci USA 100(21): 12343-12348.
- Beffara F, Perumal J, Mahyuddin AP, Choolani M, Khan SA, et al. (2020) Development of highly reliable SERS-active photonic crystal fiber probe and its application in the detection of ovarian cancer biomarker in cyst fluid. Journal of Biophotonics 13(3).
- Eom G, Hwang A, Kim H, Moon J, Kang H, et al. (2021) Ultrasensitive detection of ovarian cancer biomarker using au nanoplate sers immunoassay. BioChip J 15: 348-355.
- Perumal J, Balasundaram G, Mahyuddin AP, Choolani M, Olivo M (2015) SERS-based quantitative detection of ovarian cancer prognostic factor haptoglobin. Int J Nanomedicine 10(1): 1831-1840.
- James NE, Chichester C, Ribeiro JR (2018) Beyond the biomarker: Understanding the diverse roles of human epididymis protein 4 in the pathogenesis of epithelial ovarian cancer. Frontiers in Oncology 8: 124.
- Wang YW, Reder NP, Kang S, Glaser AK, Yang Q, et al. (2017) Raman-encoded molecular imaging with topically applied SERS nanoparticles for intraoperative guidance of lumpectomy. Cancer Research 77(16): 4506-4516.
- Chen C, Wang J, Lu D, You R, She Q, et al. (2022) Early detection of lung cancer via biointerference-free, target microRNA-triggered core-satellite nanocomposites. Nanoscale 14(22): 8103-8111.
- Kim Y, Jeong S, Jung KO, Song MG, Lee CH, et al. (2017a) Simultaneous detection of EGFR and VEGF in colorectal cancer using fluorescence-raman endoscopy. Sci Rep 7: 1035.
- Thomaidis T, Maderer A, Formentini A, Bauer S, Trautmann M, et al. (2014) Proteins of the VEGFR and EGFR pathway as predictive markers for adjuvant treatment in patients with stage II/III colorectal cancer: Results of the FOGT-4 trial. Journal of Experimental & Clinical Cancer Research 33: 83.
- Kim Y, Jeong S, Jung KO, Song MG, Lee CH, et al. (2017b) Simultaneous detection of EGFR and VEGF in colorectal cancer using fluorescence-raman endoscopy. Sci Rep 7: 1035.
- Li J, Wang C, Yao Y, Zhu Y, Yan C, et al. (2020) Label-free discrimination of glioma brain tumors in different stages by surface enhanced Raman scattering. Talanta 216: 120983.
- Phyo JB, Woo A, Yu HJ, Lim K, Cho BH, et al. (2021) Label-Free SERS analysis of urine using a 3d-stacked AgNW-glass fiber filter sensor for the diagnosis of pancreatic cancer and prostate cancer. Anal Chem 93(8): 3778-3785.
- Samriti, Rajput V, Gupta RK, Prakash J (2022) Engineering metal oxide semiconductor nanostructures for enhanced charge transfer: Fundamentals and emerging SERS applications. Journal of Materials Chemistry C 10(1): 73-95.
- Lin J, Ren W, Li A, Yao C, Chen T, et al. (2020) Crystal-amorphous core-shell structure synergistically enabling TiO2 nanoparticles’ remarkable SERS sensitivity for cancer cell imaging. ACS Appl Mater Interfaces 12(4): 4204-4211.
- Pandey A, Sarkar S, Pandey SK, Srivastava A (2022) Silica nanospheres coated silver islands as an effective opto-plasmonic SERS active platform for rapid and sensitive detection of prostate cancer biomarkers. Molecules 27(22): p. 7821.
- Liang X, Li N, Zhang R, Yin P, Zhang C, et al. (2021) Carbon-based SERS biosensor: From substrate design to sensing and bioapplication. NPG Asia Mater 13: 8.
- Lan C, Zhao J, Zhang L, Wen C, Huang Y, et al. (2017) Self-assembled nanoporous graphene quantum dot-Mn3O4 nanocomposites for surface-enhanced Raman scattering based identification of cancer cells. RSC Adv 7(30): 18658- 18667.
- Ganesh S, Venkatakrishnan K, Tan B (2020) Quantum cytosensor for early detection of cancer. Med Devices Sens 3(1): e10058
- Xi Y, Xu P (2021) Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol 14(10): p. 101174.
- Liang O, Wang P, Xia M, Augello C, Yang F, et al. (2018) Label-free distinction between p53+/+ and p53 -/- colon cancer cells using a graphene based SERS platform. Biosensors and Bioelectronics 118: 108-114.
- About glioblastoma. National Brain Tumor Society.
© 2023 Tahrima B Rouf, This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






