- Submissions

Full Text
Significances of Bioengineering & Biosciences
Metastatic Colorectal Cancer with ALK-EML4 Fusion
Pérez JC*, Román MS, Delfrade IM, Mateos RF, Puertas PR and Monteagudo RF
Department of Medical Oncology, Ramon y Cajal University Hospital, Madrid, Spain
*Corresponding author:Jesus Chamorro Pérez, Department of Medical Oncology, Ramon Y Cajal University Hospital, 28034 Madrid, Spain
Submission: January 30, 2023 Published: March 20, 2023

ISSN 2637-8078Volume6 Issue1
Abstract
Treatment with Tyrosine Kinase Inhibitors (TKIs) in patients with metastatic Colorectal Cancer (CRC) is not widespread and it is not frequent to find targetable oncogenic genetic alterations. ALK fusions exhibit a very low incidence in CRC. We present the case of a 31-year-old woman in whom an ALK-EML4 fusion was identified. After progressing to two chemotherapy-based treatments, he received a treatment sequence with alectinib and lorlatinib. Although she presented an important response at the beginning, with a significant improvement in his quality of life, the duration of the response was lesser than expected, compared to those obtained in lung adenocarcinoma with ALK fusion. Further studies are warranted to better characterize these patients and design fitting therapeutic strategies that are adapted to their particularities.
Keywords: Colorectal cancer; Genetic alterations; Chemotherapy; Adenocarcinoma
Abbreviations:TKIS: Treatment with Tyrosine Kinase Inhibitors; CRC: Colorectal Cancer; ALK: Anaplasic Lymphoma Kinase; TKIS: Tyrosine Kinase Inhibitors; CT: Computerized Tomography
Introduction
Colorectal Cancer (CRC) ranks third in terms of malignancy incidence worldwide and is the second cause of cancer mortality both in Spain and the world [1], with a median overall survival of approximately 30 months in advanced cases [2]. CRC is characterized by high heterogeneity by which mutations in oncogenes or tumor suppressor genes are the main genetic alterations responsible of its pathogenesis, while gene fusions of tyrosine kinase receptors have rarely been described. Anaplasic Lymphoma Kinase (ALK) gene fusions constitute a genetic alteration against which various Tyrosine Kinase Inhibitors (TKIs) have been designed, such as crizotinib, alectinib and lorlatinib. ALK fusions are very rare in CCR and the clinicopathological features of this entity remain unknown, as well as the sensitiveness of CRC to these TKIs.
Description of the Clinical Case
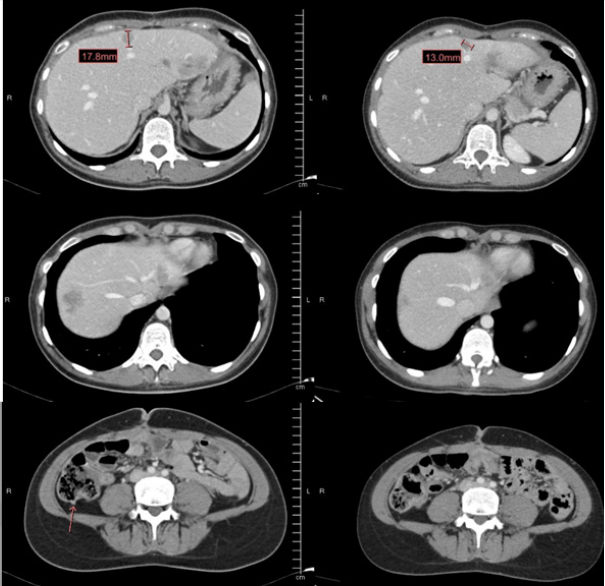
We present the case of a 31-year-old woman who underwent a routine gynecological ultrasound echography in November 2019, showing a hypoechogenic uterine nodule. A Computerized Tomography (CT) was performed, and a 5.5-centimeter neoplasm was observed in the rectum-sigma, as well as mesosigm and mesenteric metastatic nodules. Colonoscopy was then performed with biopsies compatible with colorectal adenocarcinoma with Microsatellite Stability (MSS) and wild-type KRAS, NRAS and BRAF. Tumor markers were extracted, highlighting Carcinoembryonic Antigen (CEA) 116.7ng/mL. The patient underwent surgery in February 2020 by left hemicolectomy extended to the middle rectum with resection of the mesorectum. The pathological anatomy showed moderately differentiated adenocarcinoma, with vascular permeation, free surgical edges, 8 pathological lymphatic nodes of 38 resected and one pathological right iliac lymphatic node (pT3pN2bpM1a), MSS. A Next Generation Sequencing (NGS) by Foundation One technique, highlighting an EML4-ALK fusion. In the post-surgical CT scan, hepatic and peritoneal progression was observed. Therefore, first-line systemic treatment with FOLFOX-Panitumumab was started in April 2020, receiving 10 cycles. After exhibiting a favorable response, she underwent surgery with liver resection, appendectomy and peritoneal biopsies in October 2020. Only the hepatic sample showed tumor extension. Subsequently, maintenance with 5-Fluorouracil and panitumumab in November 2020 of which she received 6 cycles. She suffered a new hepatic progression in February 2021 and received a standard second line with FOLFIRI (without bevacizumab due to portal thrombosis), with new hepatic progression in May 2021 as well as CEA elevation (344.1ng/mL). In this context, alectinib is requested for compassionate use as a third-line treatment against EML4-ALK fusion. She started alectinib 600mg every 24 hours in May 2021. A partial response of the disease is observed in the first CT scan performed in July 2021 (Figure 1) and CEA level normalized. Moreover, the patient experienced a notable improvement in her quality of life. Notwithstanding, she presented hepatic, peritoneal and lymphatic progression in December 2021. A fourth-line treatment with lorlatinib 100mg/24 hours was started, despite which after two months, the disease progressed again. Unfortunately, the patient was hospitalized due to intestinal obstruction that finally led to the patient’s death in April 2022.
Figure 1:Partial response of hepatic metastatic disease observed in CT.
Discussion
Currently, precision oncology plays a critical role in novel treatment approaches for different malignancies. The aim is to carry out tumor genomic studies in search of molecular alterations susceptible to receiving a targeted therapy against them, regardless of the histological subtype (“agnostic tumor” strategy). ALK fusions have been deeply studied in lung adenocarcinoma, achieving prolonged overall survival rates never seen before in this pathology. The frequency of oncogenic ALK fusions in patients with CRC is very rare, with an estimated frequency of 0.17-1% of cases [3,4]. It is more frequent in those patients with RAS wild-type CRC resistant to anti-EGFR treatment since it could mediate a resistance mechanism to these treatments. Fusions with genes such as EML4, STRN, CAD or C2orf44 have been described. Most of the available evidence arises from reported cases [5-8] in which an aggressive phenotype like the one shown by our patient is confirmed. Sensitivity to TKIs such as crizotinib, entrectinib, alectinib and lorlatinib has been confirmed in these reported cases, even in fusions with other genes than ALK-EML4, like STRN-ALK [7] or CAD-ALK [8]. Nonetheless, tumor responses and their duration differ considerably from those obtained in lung adenocarcinoma.
Conclusion
CRC with ALK fusions presents a different biological behavior from that observed in lung cancer. Therefore, in the same way that BRAF-mutated CRC requires a different approach from the one applied in BRAF-mutated melanoma, deep studies of these patients are warranted to identify their differential clinical and pathological characteristics as well as their particular resistance mechanisms that mediate the loss of sensitivity to TKIs, thus developing therapeutic strategies that improve their survival.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataramet I, et al. (2021) Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3): 209-249.
- Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, et al. (2017) Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: A randomized clinical trial. JAMA 317(23): 2392-2401.
- https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga
- Sheng Y, Gong FY, Wang GQ, et al. (2020) Novel ALK fusions are detected in patients with not only NSCLC, but also other solid tumors NGS fusion assay is an optional method for screening novel fusion. Presented at the ASCO.
- He X, Jiao XD, Liu K, Qin BD, Wu Y, et al. (2021) Clinical responses to crizotinib, alectinib, and lorlatinib in a metastatic colorectal carcinoma patient with ALK gene rearrangement: A case report. JCO Precis Oncol 5.
- Hsiao SY, He HL, Weng TS, Lin CY, Chao CM, et al. (2021) Colorectal cancer with EML4-ALK fusion gene response to alectinib: A case report and review of the literature. Case Rep Oncol 14(1): 232-238.
- Yakirevich E, Resnick MB, Mangray S, Wheeler M, Jackson CL, et al. (2016) Oncogenic ALK fusion in rare and aggressive subtype of colorectal adenocarcinoma as a potential therapeutic target. Clin Cancer Res 22(15): 3831-3840.
- Amatu A, Somaschini A, Cerea G, Bosotti R, Valtorta E, et al. (2015) Novel CAD-ALK gene rearrangement is drugable by entrectinib in colorectal cancer. Br J Cancer 113(12): 1730-1734.
© 2023 Pérez JC, This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






