- Submissions

Full Text
Significances of Bioengineering & Biosciences
Electrochemical Immunosensors and Biomimetic Sensors for the Detection of the Myocardial Infarction Biomarkers
Benjamin SR, Santos LKB, Silva JEG, Mendonça PD, Silva BVM, Dutra RF*
Biomedical Engineering Laboratory, Federal University of Pernambuco, Brazil
*Corresponding author: Rosa Fireman Dutra, Moraes Rego, Biomedical Engineering Laboratory, Federal University of Pernambuco, 1235. Recife, Brazil
Submission: December 19, 2022 Published: January 26, 2023

ISSN 2637-8078Volume5 Issue5
Abstract
Early and precise identification of Cardiovascular Diseases (CVDs) is crucial to preventing patients from suffering permanent damage or death, prompting the development of immunosensor devices to detect and measure cardiac biomarkers. Biomarker levels in normal and patient Electrochemical immunosensors can used to monitor serum quickly, efficiently, and affordably. The quantitative detection of cardiovascular disorders employs immunosensors based on antibodyantigen interaction. This overview elucidates Immuno and biomimetic sensors developed by detecting Acute Myocardial Infarction (AMI). The introduction summarises a background about the status of the analytical methodologies for detecting cardiac markers and the principles and limitations of the immunoassays used in laboratory routine. The conceptual topic discusses the theoretical foundation of immunosensors, emphasizing electrochemical transduction, the main alternative offered by mass production. In addition, it presents the state-of-art of electrochemical immunosensors and biomimetics for detecting important cardiac markers, troponins T and I, myoglobin, B-Type Natriuretic Peptide (BNP), and CK-MB. Finally, the challenges and prospects of cardiac marker analytical electrochemical and diagnostic methods for rapid intervention, as pointof- care testing are discussed.
Keywords: Immunosensor; Cardiac infarction; Biomimetic sensor; Point-of-care testing; Antibodyantigen
Abbreviations: CVDs: Cardiovascular Diseases; AMI: Acute Myocardial Infarction; BNP: B-Type Natriuretic Peptide
Introduction
Acute Myocardial Infarction (AMI) has the highest fatality rate among Cardiovascular Diseases (CVD). It is one of the most prevalent chronic noncommunicable diseases, posing a significant hazard to human health. World Health Organization (WHO) estimates that 23.3 million deaths will be caused by cardiovascular disorders in 2030 in developing and developed countries alike [1]. In addition to coronary heart disease, cerebrovascular disease, rheumatic heart disease, and others affecting the heart and blood vessels, cardiovascular diseases also include cerebrovascular diseases. Many factors contribute to CVD, including hypertension, obesity, genetics, smoking, and other risk factors. Early and precise identification of Acute Myocardial Infarction (AMI) may significantly lower mortality risk. The current diagnostic procedures for CVD include Electro Cardiography (ECG), chest X-ray, echo cardiography, cardiac catheterization, Cardiac Computed Tomograpy (CT), and blood testing [2]. In order to maximise the benefits of early therapy for myocardial ischemia, it is essential to have early identification of the disease. It is crucial to diagnose a patient early in order to evaluate the prognosis for the disease. According to the WHO standards, individuals must have at minimum two of the following symptoms in order to be diagnosed with cardiovascular disease: the presence of specific symptoms (chest discomfort, abnormal ECG readings, increased biochemical parameters in blood tests).
ECGs are an effective tool for directing treatment, although they are not always effective in all circumstances because some patients electrocardiograms may be normal. Therefore, the use of cardiac markers is essential in diagnosing cardiovascular disease. Biomarkers are critical in increasing disease diagnosis accuracy, risk stratification, and prognosis. Biomarkers have a number of significant properties, including (a) primary clinical specificity and sensitivity, (b) rapid biomarker release in the blood, which allows for early detection, and (c) the capacity to persist increased in the blood for a more extended period of time, and (d) the potential to be quantified [3]. Considering that the detection of myocardial injury in a patient with acute coronary syndrome requires rapid intervention, effective analytical methods for cardiac biomarkers that are portable, quick bedside, practical and economical are desirable. Cardiovascular disease biomarkers are biological analytes released into the circulation at detectable amounts throughout the onset of CVD or promptly following myocardial damage. It is widely accepted that Cardiac Troponin (cTn) is the gold standard among many biomarkers, including creatine kinase MB (CK-MB), Myoglobin (Myo), and B-Type Natriuretic Peptide (BNP) [4].
Status of analytical methods for cardiac marker
Immunoassays have been widely used in practical routines; they involve indirect measurements of the antigen-antibody reaction through chromogenic or chemiluminescent compounds. Cardiovascular troponin detection initially reported using Enzyme- Linked Immunosorbent Assay (ELISA), reported by Katus [5]. In ELISA, two monoclonal antibodies, the first troponin specific and the second peroxidase-conjugated, are used to indirectly detect cardiac troponin T by chromogens. Electrochemiluminescence Assay (ECLIA) using chemiluminescent substances like ruthenium as a labeling agent is preferred over ELISA because to its rapidity, dispensing of the enzyme reaction, and lower detection limit [6]. However, the immunoassays mentioned above represent technological advancements; they are time-consuming and need processing in a laboratory unit, restricting remote diagnostics and raising turnaround times [7]. The interval between blood collection, processing, analysis, and release of results are not supposed to exceed 60 minutes in cardiac crises [8]. The response time of medical intervention is critical and directly related to AMI’s success and therapeutic complications. The application of bedside sensing, referred to as Point-of-Care (POC) testing, is one alternative for overcoming the diagnostic delay. These devices can provide more practical, accessible, and quicker critical results, supporting the medical personnel in identifying patients at higher risk for cardiac complications and consequences, addressing an appropriate response as soon as possible, and managing clinical trials more efficiently. Recently, lateral flow strip testings for cardiac troponin T detection performed as primary triage tests in cardiac emergencies [9]. The principle of this method based on a semi-quantitative method supported by chromatography, and there is an interaction of circulating markers from the blood samples with specific antibody-conjugated to colloidal gold particles on a nitrocellulose membrane [10,11].
Despite their convenience, cheap cost, and speed, these tests have limited sensitivity and provide mainly qualitative results (yes or no as an answer). Because it is not feasible to get succinct concentrations, they are not exact and unique, as the degree of heart muscle damage is unknown. Therefore, it is crucial to promote the development of cutting-edge technology for detecting cardiac signals in AMI detection in order to shorten the duration of investigations despite maintaining their sensitivity and specificity. In recent decades, the prospect of quantitative testing with acceptable sensitivity and diagnostic specificity has increased interest in biosensor research. Biosensors can be a promising POC testing for quantitative evaluation of the cardiac injury, positively impacting early AMI diagnosis [12,13]. Biosensors are analytical devices with a biological recognition element intimately in contact with a transducer, which can convert the response of the biological recognition element and analyte into a quantifiable signal [14]. The biological recognition elements include enzymes, antigens, antibodies, cDNA, aptamers, tissues, and cells etc. Different transduction can also convert the biochemical response into a quantifiable signal, such as optical, piezoelectric, and electrochemical. Several immunosensors for detecting cardiac markers were developed using optical transduction, for instance, by surface plasmon resonance principle [15] and piezoelectric transduction using crystal quartz microbalance [15].
The optical transducer based on the Surface Plasmon Resonance (SRP) technique was the first immunosensor reported by Dutra and Kubota for cTnT detection [16]. In this optical transduction method, changes in the refractive index of light through an optical prism indicate the affinity reaction between antigens and antibodies. Optical thickness (basically refractive index) within 300nm of the surface of the diffraction component (evanescent field) governs the angle of incidence to which resonance occurs. Therefore, SPR-based sensors enable direct detection [17]. The described imunossensor reached a Limit of Detection (LOD) of 0.01ng/mL of cTnT, which is equivalent to resonant angle change of 1.28 millidegrees, and a quick response of 800s. Cardiac markers can be detected by piezoelectric transducers using Quartz Crystal Microbalances (QCM). The QCM is a very-sensitive mass sensor that can detect changes in the oscillation frequency of a quartz crystal at extremely low concentrations. Due to the piezoelectric properties of quartz, the frequency oscillation decreases proportionally with the amount of analyte adsorbed onto the electrode. Thus, the recognition reaction of the antibody with the specific antigen based on the vibration of the quartz support allows the direct detection of the analyte. Wong-Ek et al. [18] constructed a QCM sensor to detect the cTnT by utilizing Polyvinyl Chloride (PVC) doped COOH film as support for the immobilisation of the antibody against cTnT, reaching a LOD of 5ng/mL [18]. In 2012, Mattos et al. [19] developed a Dual-QCM immunosensor for detecting cTnT. The immunosensor assessed cTnT in serum samples without dilution, reaching 0.008ng/mL [19]. Although, the optical and piezoelectric immunosensors are advantageous compared to traditional immunoassays, allowing direct detection and faster analyses. Despite the fact that these systems do not have antibodies or antigens conjugated to labels, like enzymes or chemical compounds, these transduction systems are not easily transformed into a portable or low-cost technology because the gold and quartz electrodes used in optical and piezoelectric measurements, respectively, are relatively expensive for a bedside diagnostic, which should be a single-use assay. In these senses, the electrochemical transducer for mono-use immunoassay is one of the most attractive perspectives for cardiac immunosensors. Electrochemical devices have essential advantages, such as ease of miniaturisation, lower cost, and compatibility as large-scale sensor technologies facilitate point-of-care testing in the cardiac emergence department.
Electrochemical immunosensors for cardiac markers
The electrochemical immunosensors are dependent on changes in electrical properties that occur as a consequence of the interaction between antibodies and their respective antigens. Commonly, electrochemical transducers for immunoassay are (i) amperometric, measuring the electric current produced by displacement by regulated potential; (ii) potentiometric, detecting the potential difference caused by the accumulation or reduction of ions at the sensor interface when exposed to a continuous current or zero current source, e.g. Field-Effect Transistor (FET), which is a semiconductor device that operates through electric field modulating charges and can convert specific biological interactions directly into electrical signals [20,21] and (iii) impedimetric, detecting changes in the medium’s impedance based on by a current or potential disturbance on the sensor surface [15,22].
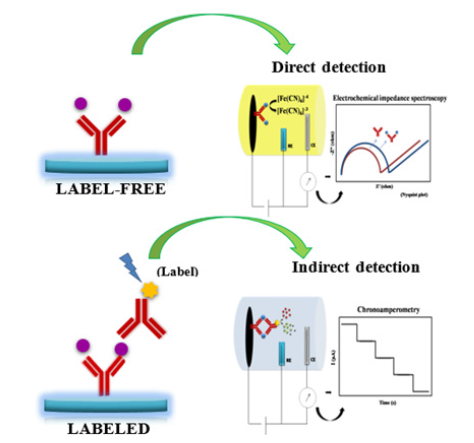
Labeled immunosensors and label-free immunosensors are two types of immunosensors (Figure 1). A labeled electrochemical immunosensor means that the antigen-antibody reaction is indirectly measured using a secondary antibody or antigen by the readout signal. This configuration of electrochemical immunoassay has a good potential for signal amplification by using electrochemical mediators and high specificity, avoiding non-specific binding. The most common labels involve enzymes, metallic nanoparticles, and electrochemiluminescent probes available in different procedures such as competitive, sandwich, and indirect assays, which require a long time and additional cost [23]. The label-free electrochemical immunosensor directly detects the antigen-antibody interaction on the electrode surface by measuring the changes in capacitance or resistance induced by the recognition event. It can be performed using different voltammetry techniques (i.e., differential pulse and square wave voltammetry-DPV, SWV), chronoamperometry, and Electrochemical Impedance Spectroscopy (EIS). Its primary benefit is the ease, speed, and cost-effectiveness of labeled immunoreagent dispensing. However, a label-free electrochemical immunosensor needs a highly selective bioreceptor for the target and a sensitive sensor surface due to the absence of a signal amplification mechanism [24,25]. The current review dealt with comprehensively detecting biomarkers of myocardial infarction using electrochemical immunosensors and biomimetic sensors.
Figure 1:Schematic illustration of the fundamentals of labeled and label-free electrochemical immunosensors.
Cardiac biomarkers
Troponin T
Troponins are a group of Three Proteins Called Troponin C (TnC), Troponin I (TnI), and Troponin T (TnT). Troponins are abundant in cardiac and skeletal muscle tissue and function in muscle contraction regulation. Cardiac Troponins I And T (cTnI and T) are cardiomyocyte-specific within the troponin complex. During an injury to the heart muscle (due to lack of oxygen and nutrients), troponins released into the circulation. Cardiac troponins (I and T) are the most sensitive and specific indicators for AMI diagnosis and prediction [26] Cardiac Troponin T (cTnT), Cardiac Troponin I (cTnI) are “gold-standard” markers in the AMI diagnostic. cTnT levels in peripheral blood rise within 3-4 hours after the initial myocardial infarction and stay elevated for 10-14 days after the injury. The first biosensor for cTnT was developed by our group in 2007 [16], which was based on optical transduction and allowed a label-free detection of cTnT in serum samples. Considering that this transducer type is challenging to miniaturise due to the high power consumption and instability by mechanical and thermal changes, other types were also attempted, including the piezoelectric [19] and capacitive immunosensors [27]. However, the requirement of a very low detection limit by cTnT for AMI diagnostic made them still not satisfactory immunosensors.
Nevertheless, the most developed transducer is amperometric; one essential advantage is the ease of mass-production and miniaturisation by tips. In 2010, our group proposed the first electrochemical immunosensor for cTnT diagnosis, condtructed on a streptavidin-microsphere modified disposable Screen-Printed Electrode (SPE) [28]. The strong affinity interaction between microsphere streptavidin and anti-cTnT biotin-conjugated allowed increased analytical sensibility to cTnT detection in order of nanograms. The antigen-antibody reactions were quantified indirectly by using a second antibody, peroxidase-conjugated. This immunosensor demonstrated a linearity range up to 0.1-10ng/mL and a low detection limit of 0.2ng/mL [29]. Novel nanomaterials, including Metal Nanoparticles (MNs), Graphene (G), Carbon Nanotubes (CNT), Conductive Polymers (CP), surfactants, and liquid crystals, have been used to construct immunosensors with high troponin detection sensitivity. Recently, Gomes-Filho et al. [30] reported the introduction of the nanomaterials on an imunosensing platform for cTnT detection in human blood samples. The excellent conductivity and high surface/ratio of the CNTs were employed to increase the immobilised anti-cTnT antibody. The sensor surface is composed of nanocomposite thin films of polyethyleneimine and carboxylated CNTs (Figure 2). The LOD achieved for this sensor was 0.033ng/mL with a linear response range between 0.1 to10ng/mL cTnT. However, the indirect measurement using labelled secondary antibodies with horseradish peroxidase demands many incubation steps, and the sensor has shown great sensitivity and decreased LOD [30].
Figure 2: Stepwise of a labelled immunosensor construction by using carbon nanomaterial. Illustration adapted from [30] representing the immunosensor construction based on a nanocomposite thin-film of poly ethyleneimine and carboxylated CNTs.

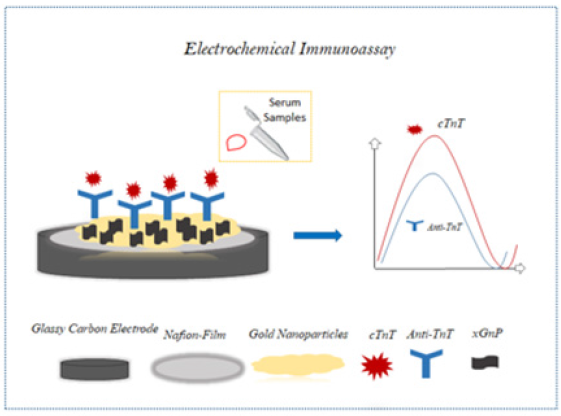
In order to develop direct detection technology with the potential for point-of-care diagnostic using a SPE, Silva et al. [31] designed a label-free electrochemical immunosensor. A polyethylene terephthalate substrate used to form SPE based on a carbon ink containing amino-functionalised CNTs. CNT incorporated in the printed film improved the charge transfer rate due to its electrical proprieties. The amine groups derived from the CNT was employed to avoid non-random immobilisation of the anti-cTnT. A low LOD was achieved (0.0035ng/mL), superior to conventional analytical methods [30]. Over the years, researchers aimed to develop sensors with lower LOD and high sensibility., furthermore, Shanmugam and colleagues [32] developed the electrochemical immunosensor based on inorganic zinc oxide nanostructures to enable confinement of biomolecules, increasing the signal from the biosensor. In addition to the semiconductive properties of ZnO, it was also used to improve the sensitivities of the sensor. The sensor response characterised by the combination of AC and DC spectroscopy that provided the development of devices able to be used in cardiac emergencies because of their ability to reveal biomolecules in the order of pg/mL. However, AC and DC spectroscopy is unsuitable for mass production since it requires a complex system. Recently, Zanato et al. [33] presented the synergistic impact of exfoliated Graphite Nanoplatelets (xGnP) coated with gold Nanoparticles (AuNP) for detecting and measuring cTnT samples of human blood plasma using electrochemical immunosensors (Figure 3). In contrast to other nanomaterials like carbon nanotubes, xGnP structures have cheap cost, large surface area, and electrical conductivity. Additionally, the synergism between xGnP/AuNP results in a hybrid material with minimised limitations related to self-agglomerations. The immunosensor assembly was performed on a glassy carbon electrode with nafion film, that deposited in a solution of anti-TnT and AuNP-Hep- xGnP. Real human blood plasma samples with cTnT tested on the immunosensor platform, which achieved a linear range between 0.050 to 0.35ng/mL and detection limit of 0.016ng/ mL of cTnT. Despite this immunosensor not presenting a detection limit higher than the existing ones, a good accuracy (<10%) was obtained for interference in blood plasma, demonstrating an advance in recognition of biomolecules in complex matrices.
Figure 3:Electrochemical immunoassay for cTnT detection in human blood plasma. illustration adapted from [33] representing an immunosensor construction based on a solution of anti-TnT and AuNP-HeP- xGnP glassy carbon electrode with nafion film.
Troponin I
Cardiac Troponin I (cTnI) is a protein with a Molecular Weight (MW) of 29kDa that inhibits the troponin-tropomyosin complex. cTnI is released quickly into the circulation after 3-6 hours following AMI injury to cardiac muscle tissue and peaks within 24-48 hours. In general, values return to baseline 5-14 days following the onset of an injury. Serum cTnI levels are directly connected to the risk of mortality for patients who have been infracted, and because of its clinical value in the identification of cardiac diseases, specific biosensors have been developed for detecting this biomolecule [34]. Initially, Ko et al. [35] constructed a microchip that used an Interdigitated Array (IDA) gold electrode to detect the cTnI through a secondary antibody linked to an alkaline phosphate enzyme. PDMS channels were constructed ton IDA chips to facilitate the transfer of electrons from the surface of the gold electrode to the electroactive species formed by enzymes. Finally, anti-cTnI was attached to the layer of protein-G via silanisation on the PDMS channel inside the surface. Immunosensor orientation and sensitivities improved by the specific binding of the IgG antibodies to protein G, which interacts with the Fc region of IgG antibodies.
The electrochemical immunosensor detected cTnI at concentrations ranging from 0.2ng/mL -10g/mL with excellent sensitivity. Surfacefunctionalized PDMS channels were employed for antibody orientation by protein G bound to gold electrodes, resulting in a lower limit of detection (LOD) of 148pg/mL. Five years later, Kong et al. [36] demonstrated a Silicon Nanowire (SiNW) based Field-Effect Transistor-(FETs) based label-free biosensor for cTnI detection. In order to build the device using CMOS technology, top-down design was used. It is possible to mass-produce miniaturised sensors for commercialisation utilising this technology. Anti-cTnT monoclonal antibody was covalently immobilised on SiNW previously silanised with 3-Aminopropyl Triethoxysilane (APTES), using aldehyde groups as covalent immobilisers. The immunosensor was very sensitive to cTnI, detected at 0.092ng/mL LOD. The suggested platform has significant mass-production potential for sensors used in AMI clinical diagnostics. Wang et al. [37], who developed the label-free cTnI biosensor, used an electrochemical impedance spectroscopy-based method for detection. This study employed a biorecognition molecule based on the structure of cTnI. The biosensor was made by electrodepositing Gold Nanoparticles (GNPs) on a typical glassy carbon electrode prior to thiol selfassembling the peptide probe. The biosensor’s charge transfer resistance (Rct) was measured in relation to the concentration of cTnI associated with the transfer of electrons from the redox pair [Fe(CN)64/3]. The biosensor demonstrated a reduced Limit of Detection (LOD) (3.4pg/mL) compared to earlier experiments. The biosensor for cTnI based on peptides also showed good selectivity in various interferents (AFP, IgG, BSA, and Albumin).
Dendrimer (Den) was used as an innovative immunosensor device by Akter et al. [38] to detect cTnI in blood samples without needing labels or reagents. The immunosensor was constructed by covalently connecting carboxylic acid (COOH)-functionalised third Generation (G3) poly (amidoamine) dendrimer (Den) to the 3, 3′, 5, 5′-Tetramethylbenzidine (TMB) Modified 6-Mercaptohexanoic Acid (MHA) Self-Assembled Monolayer (SAM) on A Gold Electrode (Au). The monoclonal anti-cardiac troponin I antibody (anticTnI) was immobilised to the electrode surface by an affinity for poly (amidoamine) (PAMAM) and Den. The authors used the Den to immobilize the highest proportion of antibodies on the sensor surface as a way to improve the sensitivity of electrochemical signals. The characteristics of the immunosensor were examined using XPS, QCM, CV, and EIS. Consequently, Den can enable a labelfree immunosensor to detect troponin in blood samples at low levels (11.7fM). The analytical performance of the immunosensor was stable and specific, and it showed good agreement with ELISA techniques.
B-type natriuretic peptide (BNP): The B-type natriuretic peptide is utilized to ensure the indication of acute myocardial infarction. During pressure overload or volume expansion of the left ventricular myocardium, the substance is released into the bloodstream. The significance of BNP in diagnosing and prognosizing acute heart failure has led researchers to develop simple, specific biosensors for BNP detection [39]. According to Matsumura et al. [40], the electrochemical enzyme immuno-assay system detect BNP in a wide dynamic linear range of 20 to 100ng/L and a detection limit of 10ng/L. In this experiment, serum samples added to anti-BNP labelled with Acetylcholinesterase (AChE). This study uses the electrochemical enzyme immunoassay method to determine AChE as the most sensitive method to increase BNP detection. This chemisorption/electrochemical desorption of thiocholine on a silver electrode is the mechanism that allows this electrochemical enzyme immunoassay system to modulate AChE activity. Consequently, the cost-effectiveness kit with an appropriate LOD was constructed compared to commercial kits. Lee et al. [41] demonstrated label-free conductometric sensors using single PANINWs and microfluidic channels to measure the concentration of 100pg/mL(Myo), 250fg/mL(cTnI), 150fg/mL(CK-MB), and 50fg/ mL(BNP). The electrodeposition used to fabricate PANI-NWs on patterned Au electrode. This technique utilised patterning on the surface of Au electrodes to allow for the controlled development of PANI-NWs within pattern direction Myocardial Antibodies (mAbs) used to covalently functionalised PANI-NWs in the biosensor. The conductometric sensing approach is employed to identify cardiac biomarkers, which detect variations in nanowire conductance. Following the addition of target biomarkers to Phosphate Buffer (PBS) Solution (pH 7.4), the immobilised mAbs and biomarkers bind, resulting in a shift in the conductance of a single PANi-NW. The biosensor selectivity was tested using microfluidic channels and bovine serum albumin. The designed biosensor was 106 times more specific for cardiac biomarkers than a BSA.
Recently, Shanmugam et al. [42] produced electrochemical sensor for BNP, cTnI, and cTnT detection. The use of lowtemperature hydrothermal procedures enabled the development of a label-free combined sensor with POC device using anisotropic ZnO nanostructures. The analytical measurement was performed by impedance spectroscopy and Mott-Schottky electrochemical techniques. This biosensor showed a linear response in human serum tests of 1pg/mL-100ng/mL and LOD of1pg/mL.
CK-MB
CKMB is one of the three Creatine Kinase (CK) enzyme forms. MB isoenzyme is present in cardiac muscle; its determination should be specific to diagnose myocardial infarction and myocarditis. Electrochemical sensors for measuring creatine kinase based on enzymatic processes. Taking into consideration the growth of approaches used in the design of biosensors, Moreira et al. [43] describe a new method for CK-MB measurements. Initially, AUSPE (screen-printed electrode) was used covered by cysteamine (Cys). Then, the phosphorylated form of creatine (Pcrea) was immobilised on previously electrode modified. The use of Pcrea was crucial due to its electroactive behaviour in low potential and ability to bind to CK-MB. As a result, it was possible to detect CK-MB directly in a system label-free, which demonstrated good selectivity front interference studies realised with cardiac troponin (TnT), Myoglobin (Myo), bovine serum albumin (BSA). The LOD obtained was 0.11mg/mL. Cen et al. [44] identified creatine kinase MB in serum samples using ultrathin AuPdCu alloyed nanowire-like networks (NWNs). The ultrathin AuPdCu NWNs were accomplished by utilizing an eco-friendly one-pot aqueous technique including 4-aminopyridine. The label-free electrochemical immunosensor for CK-MB immunoassay was fabricated effectively due to the enhanced surface area and abundance of active sites produced by the AuPdCu NWNs. The constructed immmunosensor exhibited a broad linear range between 0.001-2000ng/mL for CK-MB detection limit of 0.88pg/mL. Sandwich-type electrochemical immunosensors are employed more in clinical practice than label-free immunosensors, owing to their improved selectivity, sensitivity, low background intensity, and wide dynamic range. Recently, Yang and his coworkers [45] built the sandwich-type electrochemical immunosensor for the immunoassay of CK-MB was successfully developed using porous PdPtCoNi@Pt-skin NP wrapped Thi and Au NSs as a sensing platform. The electrochemical immunosensor was able to detect cTnT levels in human blood samples with a LOD of 0.62pg/ml and a detection range of 0.001-2500 ng/mL. In another work, Kucherenko et al. [46] proposed a biosensor to measure CK activity in actual serum samples, which can be reusable. Amperometric transducer based on platinum disk electrodes with surface-deposited glucose oxidase and hexokinase. Creatine kinase’s enzymatic response governs these enzymes’ biological activity. The simplicity of this work’s sensor design is mainly owing to the one-step electrode surface preparation. In addition, the biosensor is used to determine other kinases in medical analysis of serum blood. It achieved a good performance without interferences in CK biorecognition.
Myoglobin
Myoglobin (Myo=muscles) is a heme protein responsible for transporting oxygen in cardiac muscle and is found to be exceptionally high 1 hour after an ischemic event [47]. The identification of Myo, a non-enzymatic protein biomarker of heart injury and one of the first markers of acute myocardial infarction, in the blood rapidly is essential for diagnostic techniques. The rapid elevation of Serum Myo permits early identification of the AMI, which is considered a strategic marker for POCs testing. Over the years, several researchers have published involving immunosensor for myoglobin identification [48-52]. According to O’Regan et al. [53] an amperometric immunosensor using a disposable screenprinted electrode can be developed using an indirect sandwich assay as described in a one-step technique. Polyclonal goat antihuman cardiac myoglobin, monoclonal mouse anti-myoglobin, and goat anti-mouse IgG coupled to Alkaline Phosphatase (AP) linked to alkaline phosphatase were used in this experiment. Also, The ampermoetric immunosensor was reported to have a linear detection range of 85-925pg/ml with a LOD of 0.4pg/ml to measure the cTnT levels in spiked human whole blood samples.
The recent work, Singh and coworkers [51] described an electrochemical immunosensor based on AuNPs@rGO carboxylated CNTs covered by a conductive polymer film of Polyethyleneimine (PEI) based on in-situ Screen-Printed Electrode (SPE) for the detection of cardiac biomarker myoglobin in serum samples. The prototype electrochemical immunosensor showed a low detection limit of 0.67ng/mL and a wide linear detection range of 1 to 1400ng/mL for the Myo concentration. The limit of detection for electrochemical-based detection is 0.67ng/mL, which is substantially better than the 4ng/mL reported with ELISA assays employing the same antibodies. Potential future breakthroughs include using MIP; nanostructure semiconducting interdigitated nanoelectrodes for incredibly flexible and efficient sensors. Interdigitated Array Electrodes (IDA) enable label-free detection using impedance spectroscopy and may be used with microfabricated devices made in batches. Microfluidic channels were combined with polyaniline nanowires to detect low concentrations of cardiac biomarkers [41]. Sharma et al. [49] developed a carbon 3D system-based immunosensor made up of a suspended mesh and IDA nanoelectrodes to amplify signals for Cardiac Myoglobin (cMyo) detection. The proposed method allowed for effective redox cycles and linear detecting ranges of 0.001-10ng/mL with a calculated LOD of 0.43 pg/mL. (Table 1) summarises recent investigations using electrochemical techniques for Myo detection.
Other cardiac markers
Other cardiac biomarkers, such as Lactate Dehydrogenase (LDH) and the human Heart Fatty Acid-Binding Protein (h-FABP), have been investigated as electrochemical immunosensors [54,55]. Despite the scarcity of studies, the clinical characteristics of AMI still need to be highlighted.
Biomimetic Sensors to Cardiac Markers
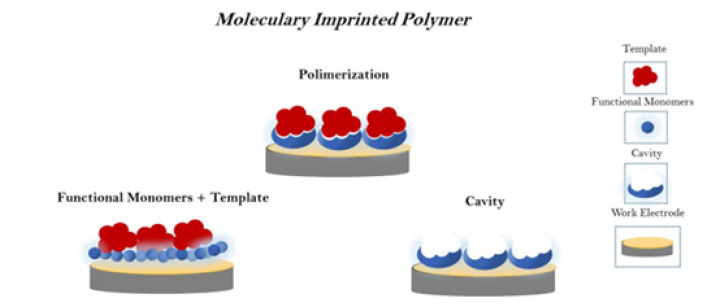
Biomimetic sensors based on Molecular Imprinting Polymers (MIPs) are promising for fabricating polymeric biomimetic receptors. The analyte detection is dependent on the template concentration adsorbed by a MIP. In the last decade, a significant number of researchers have developed MIPs to detect cardiac markers. Over the years, they have become more technologically sophisticated but with a simple design. Conventional techniques of obtaining MIPs (i.e. in bulk) usually need an exhaustive study for optimisation, the difficulty of immobilisation on the sensor surface, and high manufacturing cost. The following compounds form the MIPs: a target molecule, which serves as a template in the printing; functional monomers; binding reagents, which contribute to the formation of the polymer chain and a solvent used in the template removing [14]. It allows the construction of polymeric structures in 3D with similar receptor or catalytically active sites (Figure 4). Biomimetic cardiac marker sensors manufactured using a range of plastic antibodies, emphasizing troponins, myoglobins, and others. The elevated high-sensitivity cardiac troponins in myocardial injury and their reduced molecular size have attracted research to develop the biomimetic sensor. Moreira et al. [56] described the first study that developed a sensitive biomimetic sensor for cTnT. N, N′-methylenebisacrylamide (acrylamide) used as the functional monomer and ammonium persulfate used as the initiator in the fabrication of this platform. The biomimetic sites assembled onto the nanostructured surface of carboxylated multi-waled carbon nanotubes. Potentiometric transduction methods detected CTnTMIP binding events. The Non-Molecularly Imprinted Polymer (NIP), obtained by imprinting without cTnT, showed the non-rebindingability that confirms the selectivity of the cTnT-MIP. The MI-based sensors measured TnT in artificial serum samples linear ranging from 1.41-20.86g/mL, with a limit of detection 0.16g/mL. In 2013, Karimian et al. [56] designed an excellent biomimetic sensor with low LOD of cTnT by using o-phenylenediamine (o-PD) as functional monomers on a gold electrode. The study described obtaining a biomimetic site by employing electro synthesised MIP directly on the sensor surface as a thin film. CV, EIS, and atomic force microscopy characterised the imprinted biomimetic sites. This sensor exhibited a lower LOD of 9pg/mL of cTnT, similar to the cutoff obtained by traditional methods of cTnT dosage.
Figure 4:Principle of biomimetic sensors constructed through functional monomers submitted to polymerisation technique in order to form cavities able to detect biomolecules.
Additionally, Silva et al. [57] demonstrated innovative detection results of cTnT in serum samples using a screen-printed electrode mounted on a nano-molecularly imprinted polymer. The CV and DPV techniques characterise the step production of the biomimetic surface, with the K(Fe(CN)6 3/4 serving as a redox probe. The ultrasensitive nanostructured MIP-sensor showed a very low LOD (0.006ng/mL) of cTnT in serum samples. Conducive polymer and graphene working in tandem to build three-dimensional structures with reactive groups led to a MIP with an exceptional affinity for cTnT binding. In addition, Zuo et al. [58] established a new electrochemical MIP-sensor to monitor the cTnI based on O-aminophenol as a polymeric matrix. The proposed cTnI-MIP detected the concentration of cTnI in Serum without requiring extensive sample preparation. The authors used CV to evaluate polymer electro polymerisation and the EIS technique to characterise MIP. This MIP- based sensor presents advantages including great sensibility, high specificity, quick response, low cost of preparation, and label-free determination. The LOD found to be 0.027nM processed in just 5 minutes. Moreira et al. [59] reported a sensitive plastic antibody for Myo detection constructed on a polymeric layer of carboxylated poly vinyl chloride (PVC COOH). The MIP-sensor was based on the electrochemical transducer using impedance and square wave voltammetry as detection methods. The Myo MIP achieved a LOD of 2.25g/mL with ease of design, short reaction time, excellent precision, and good accuracy. The same research group developed a new Myo biomimetic sensor based on a PVC matrix and the plasticiser o-nitrophenyloctyl ether assembled on graphite support. The lower LOD of 0.79μg/mL of Myo by using the SWV method.
Moreira et al. [60] developed a Myo MIP-based on the surface imprinting method in another approach. Myo absorbed a gold screenprinted electrode, and aminophenol monomers electropolymerized to produce a biomimetic conductive layer. In order to improve the selection of the biomimetic cavities, the peptide bonds Myo in the MIP layer were removed through the specific hydrolyse reaction of the proteinase K. The biomimetic sensor demonstrated the integration of the MIP directly on a sensor surface by using a simple electrochemical procedure of electrosynthesis. Recently, Phonklam [61] analysed the electrochemical biosensor based using a modifie glass carbon electrode with MWCNTs and Poly-Methylene Blue (PMB) to increase the electroactive surface area for cTnT binding and also to enhance the detection of redox reactions. The linear range of 0.10-8.0pg/mL was observed for the PMB/MWCNTs sensor, with a LOD of 0.040pg/mL for detecting cTnT levels in human blood.
Summary of Cardiac Marker Biosensors
The detection of cardiac markers demonstrated significant importance in diagnosing Cardiovascular Diseases (CVD). Electrochemical sensing elaboration can help fast detect these proteins with low concentrations and low time-consuming required in routine clinical. (Table 1), [62] summarises the main electrochemical immunosensor described in this chapter in the last years [63].
Table 1:Summary of electrochemical immunosensor development for cardiac markers. 1Interdigitated Array, 2Poly(dimethyl siloxane), 3Glassy carbon electrode, 4Gold nanoparticles, 5Poly(amidoamine), 6Dendrimer, 73,3’,5,5’-tetramethyl benzidine, 86 Mercaptobenzoic acid, 9Self-assembled monolayer, 10Polyethyleneimine, 11Carbon nanotube, 12Chitosan, 13Cysteamine,14Glucose oxidase, 15Carboxylated poly(vinyl chloride), 16Graphene quantum dots, 173-amino propyl trimethoxy silane/ indium-tin-oxide, 18poly(pyrrole-co-pyrrolepropylic acid, 19o-phenylenediamine, 20O-aminophenol, 21polymethylene blue, 21Graphene sheets/tin dioxide and Zinc Cobalt Oxide/N-doped carbon nanotubes, 22homogenous ordered self-assembled monolayers/capacitive interdigitated immunosensor, 23MXene: Transition metal carbide or nitride, hc-g-C3N4@CDs- Core-shell high-crystalline graphitic carbon nitride@carbon dots, 24Au NDs/Chit-g-Fc-Au nano dendrites/chitosangrafted- ferrocene, 25MUA-GE-11-mercaptoundecanoic acid, gold electrode, 26AuNPs-S-Phe-SPCE -Gold nanoparticles- 4-aminothiophenol-Screen printed carbon electrode, AuNCs-Gold nanochains, 27carboxylic-modified magnetic beads.

Conclusion and Future Perspectives
In clinical testing, biosensors, particularly immunosensors, are useful because they rely on compassionate and precise antigen-antibody responses. Additionally, [64] they play a crucial function in determining the concentration of certain substances in biological matrices, such as blood and plasma. The electrochemical immunosensor is reliable, low-cost, easy to operate, and offers quick response times. This review illustrates the recent developments in the field of voltammetric immunosensors for the detection and monitoring of several cardiac biomarkers in AMI. The development of immunosensors capable of detecting these biomarkers in a suitable method is essential to saving lives and decreasing treatment costs. The immobilization of the antibody is a crucial part of building immunosensors since antibodies are the recognition element in antibody-antigen responses. However, using the suitable antibody surface, the capacity of the antibody to bind antigen can be increased [65-70]. Consequently, the immobilisation of antibodies is crucial to the construction of an immunosensor [71- 73]. The physical and chemical adsorption of antibody molecules on the transducer surface can be used to develop oriented antibody molecules. The sensitivity of a biomarker is an essential quality for its use in the diagnosis of diseases at an early stage. In addition, electrode materials combined with nanostructured materials (e.g. silicon nanowires, gold nanoparticles, carbon nanotubes, magnetic particles, and quantum dots) can allow for the multiplexing of biomarkers . Furthermore, using multifunctional nanomaterials with a wide range of nanostructures could enhance the sensor’s performance, sensitivity, and accuracy. Moreover, the repeatability and stability are at risk because of the difficult preparation procedure and the complexity of the immunoassay [74-76]. However, the integration of microfluidic and paper-based platforms as well as hand-held products, arrays, and chips can be accomplished via high degree of automation, accessiblicity and miniaturisation, opening the way for the deployment of devices in the near term.
The significant challenges are the necessity of rigorous validation studies for direct measurement of cardiac markers in biological fluids (blood) without preparations of samples and online results [77-79]. Additionally, the incorporation of a new sensing platform with a sensor reveals intriguing possibilities for tailored POC assessment with low-cost and portable technology.
Acknowledgement
The authors gratefully acknowledge the financial support of the Brazilian National Council for Scientific and Technological Development of Brazil, grant number 440605/2016-4 and the CAPES-Coordination for the Improvement of Higher Education Personnel, Brazil (Grant number 88881.130797/2016-01),and National Institute of Science and Technology in Bioanalytics (INCTBio).
References
- Alwan A (2011) Global status report on noncommunicable diseases 2010. World Health Organization, Switerzland.
- Kakoti A, Goswami P (2013) Heart type fatty acid binding protein: structure, function and biosensing applications for early detection of myocardial infarction. Biosens Bioelectron 15(43): 400-411.
- (2014) World Health Organization, WHO. Global status report on noncommunicable diseases, Switzerland.
- Babuin L, Jaffe AS (2005) Troponin: the biomarker of choice for the detection of cardiac injury. Can Med Assoc J 173(10): 1191-1202.
- Katus HA, S Looser AR, Hallermeier K, Scheffold, T, Kübler W (1989) Enzyme linked immuno assay of cardiac troponin T for the detection of acute myocardial infarction in patients. J Mol Cell Cardiol 12:1349-1353.
- Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, et al. (2010) Analytical validation of a high-sensitivity cardiac troponin t assay. Clin Chem 56(2): 254-261.
- Elisa JR (1995) Theory and practice. Methods in molecular biology Clifton N J 46(1): 218.
- arquivosonline.com.br.
- Koczula KM, Gallotta A (2016) Lateral flow assays. Essays Biochem 60(1): 111-120.
- Bahadır EB, Sezgintürk MK (2016) Lateral flow assays: Principles, designs and labels. TrAC-Trends Anal Chem 82: 286-306.
- Christenson RH, Azzazy HME (2009) Cardiac point of care testing : A focused review of current national academy of clinical biochemistry guidelines and measurement platforms. Clin Biochem 42(3): 150-157.
- Florkowski C, Don-Wauchope A, Gimenez N, Rodriguez-Capote K, Wils J, et al. (2017) Point-of-care testing (POCT) and evidence-based laboratory medicine (EBLM)-does it leverage any advantage in clinical decision making? Crit Rev Clin Lab Sci 54(7-8): 471-494.
- Sarangadharan I, Wang SL, Sukesan R, Chen P, Dai TY, et al. (2018) Single drop whole blood diagnostics: portable biomedical sensor for cardiac troponin detection. Anal Chem 90(4): 2867-2874.
- Moina C, Ybarra G (2011) Fundamentals and applications of immunosensors.
- Grieshaber D, MacKenzie R, Vörös J, Reimhult E (2008) Electrochemical biosensors-sensor principles and architectures. Sensors 8(3): 1400-1458.
- Dutra RF, Kubota LT (2007) An SPR immunosensor for human cardiac troponin T using specific binding avidin to biotin at carboxymethyldextran-modified gold chip. Clin Chim Acta 376(1-2): 114-120.
- Altintas Z, Fakanya WM, Tothill IE (2014) Cardiovascular disease detection using bio-sensing techniques. Talanta 128: 177-186.
- Wong-ek K, Chailapakul O, Prommas J, Jaruwongrungsee K, Nuntawong N, et al. (2009) QCM based on flow system for cardiovascular disease pp. 80-83.
- Mattos AB, Freitas TA, Silva VL, Dutra RF (2012) A dual quartz crystal microbalance for human cardiac troponin T in real time detection. Sensors and Actuators B: Chemical 161(1): 439-446.
- Yin PT, Shah S, Chhowalla M, Lee KB (2015) Design, synthesis, and characterization of graphene-nanoparticle hybrid materials for bioapplications. Chem Rev 115(7): 2483-2531.
- De Moraes A, Kubota L (2016) Recent trends in field-effect transistors-based immunosensors. Chemosensors 4(4).
- Bakker E (2004) Electrochemical sensors. Analytical Chemistry 76: 3285-3298.
- Rapp BE, Gruhl FJ, Länge K (2010) Biosensors with label-free detection designed for diagnostic applications. Analytical and Bioanalytical Chemistry 398: 2403-2412.
- Lindholm-Sethson B, Nyström J, Malmsten M, Ringstad L, Nelson A, et al. (2010) Electrochemical impedance spectroscopy in label-free biosensor applications: Multivariate data analysis for an objective interpretation. Analytical and Bioanalytical Chemistry 398: 2341-2349.
- Paleček E, Dorčák V (2017) Label-free electrochemical analysis of biomacromolecules. Applied Materials Today 9: 434-450.
- Takeda S, Yamashita A, Maeda K, Maéda Y (2003) Structure of the core domain of human cardiac troponin in the Ca2+-saturated form. Nature 424(6944): 35-41.
- V EAD, Peres NG, Pereira CO, Da Silva VL, Da Silva, et al. (2009) Potential of a simplified measurement scheme and device structure for a low-cost label-free point-of-care capacitive biosensor. Biosens Bioelectron 25(4): 870-876.
- Silva BVM, Cavalcanti IT, Mattos AB, Moura P, Sotomayor MDPT, et al. (2010) Disposable immunosensor for human cardiac troponin T based on streptavidin-microsphere modified screen-printed electrode. Biosens Bioelectron 26(3): 1062-1067.
- Silvaa BVM, Cavalcantia IT, Mattosa AB, Mouraa P, Sotomayor MDPT, et al. (2010) Disposable immunosensor for human cardiac troponin T based on streptavidin-microsphere modified screen-printed electrode. Biosensors and Bioelectronics 26(3): 1062-1067.
- Gomes-Filho SLR, Dias ACMS, Silva MMS, Silva BVM, Dutra RF (2013) A carbon nanotube-based electrochemical immunosensor for cardiac troponin T. Microchem J 109: 10-15.
- Silva BVM, Cavalcanti IT, Silva MMS, Dutra RF (2013) A carbon nanotube screen-printed electrode for label-free detection of the human cardiac troponin T. Talanta 117: 431-437.
- Shanmugam NRNR, Muthukumar S, Selvam APAP, Prasad S (2016) Electrochemical nanostructured ZnO biosensor for ultrasensitive detection of cardiac troponin-T. Nanomedicine 11(11): 1345-1358.
- Zanato N, Talamini L, Zapp E, Brondani D, Vieira IC (2017) Label-free electrochemical immunosensor for cardiac troponin T based on exfoliated graphite nanoplatelets decorated with gold nanoparticles. Electroanalysis 29(7): 1820-1827.
- Wu AHB, Feng YJ, Moore R, Apple FS, Mcpherson PH, et al. (1998) Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I. American Association for Clinical Chemistry Subcommittee on cTnI Standardization. Clin Chem 44(6): 1198-1208.
- Ko S, Kim B, Jo SS, Oh SY, Park JK (2007) Electrochemical detection of cardiac troponin I using a microchip with the surface-functionalized poly(dimethylsiloxane) channel. Biosens Bioelectron 23(1): 51-59.
- Kong T, Su R, Zhang B, Zhang Q, Cheng G. (2012) CMOS-compatible, label-free silicon-nanowire biosensors to detect cardiac troponin i for acute myocardial infarction diagnosis. Biosens Bioelectron 34(1): 267-272.
- Wang B, Jing R, Qi H, Gao Q, Zhang C. (2016) Label-free electrochemical impedance peptide-based biosensor for the detection of cardiac troponin I incorporating gold nanoparticles modified carbon electrode. JEAC 781: 212-217.
- Akter R, Jeong B, Lee YM, Choi JS, Rahman MA (2017) Femtomolar detection of cardiac troponin I using a novel label-free and reagent-free dendrimer enhanced impedimetric immunosensor. Biosens Bioelectron 91: 637-643.
- Maalouf R, Bailey S (2016) A review on B-type natriuretic peptide monitoring: Assays and biosensors. Heart Fail Rev 21(5): 567-578.
- Matsuura H, Sato Y, Niwa O, Mizutani F (2005) Electrochemical enzyme immunoassay of a peptide hormone at picomolar levels. Anal Chem 77(13): 4235-4240.
- Lee I, Luo X, Huang J, Cui XT, Yun M (2012 )Detection of cardiac biomarkers using single polyaniline nanowire-based conductometric biosensors. Biosensors 2(2): 205-220.
- Shanmugam NR, Muthukumar S, Tanak AS, Prasad S (2018) Multiplexed electrochemical detection of three cardiac biomarkers cTnI, cTnT And BNP using nanostructuredZnO-sensing platform. Future Cardiol 14(2): 131-141.
- Moreira FTC, Dutra RAF, Noronha JP, Sales MGF (2014) Novel sensory surface for creatine kinase electrochemical detection. Biosens Bioelectron 56: 217-222.
- Cen SY, Feng YG, Zhu JH, Wang XY, Wang AJ, et al. (2021) Eco-friendly one-pot aqueous synthesis of ultra-thin AuPdCu alloyed nanowire-like networks for highly sensitive immunoassay of creatine kinase-MB. Sensors Actuators, B Chem 333: 129573
- Wang XY, Chen Y, Mei LP, Wang AJ, Yuan PX, et al. (2020) Confining signal probe in porous pdptconi@pt-skin nanopolyhedra to construct a sandwich-type electrochemical immmunosensor for ultrasensitive detection of creatine kinase-mb. Sensors Actuators B Chem 315: 128088.
- Kucherenko IS, Soldatkin OO, Lagarde F, Jaffrezic-Renault N (2015) Determination of total creatine kinase activity in blood serum using an amperometric biosensor based on glucose oxidase and hexokinase. Talanta 144: 604-611.
- De Winter R, Lijmer JG, Koster RW, Hoek FJ Sanders JT (2000) Diaccuracy of myoglobin concentration for the early diagnosis of acute myocardial infarction. Ann Emerg Med 35(2):113-120.
- Karami P, Bagheri H, Johari-Ahar M, Khoshsafar H, Arduini F, et al. (2019) Dual-modality impedimetric immunosensor for early detection of prostate-specific antigen and myoglobin markers based on antibody-molecularly imprinted polymer. Talanta 202: 111-122.
- Sharma D, Lee J, Shin H (2018) An electrochemical immunosensor based on a 3D carbon system consisting of a suspended mesh and substrate-bound interdigitated array nanoelectrodes for sensitive cardiac biomarker detection. Biosens Bioelectron 107: 10-16.
- Tuteja SK, Chen R, Kukkar M, Song CK, Mutreja R, et al. (2016) A label-free electrochemical immunosensor for the detection of cardiac marker using graphene quantum dots (GQDs). Biosens Bioelectron 86: 548-556.
- Singh S, Tuteja SK, Sillu D, Deep A, Suri CR (2016) Gold nanoparticles-reduced graphene oxide based electrochemical immunosensor for the cardiac biomarker myoglobin. Microchim Acta May 183(5): 1729-1738.
- Ren X, Zhang Y, Sun Y, Gao L (2017) Development of electrochemical impedance immunosensor for sensitive determination of myoglobin. Int J Electrochem Sci 12: 7765-7776.
- O’Regan TM, O’Riordan LJ, Pravda M, O’Sullivan CK, Guilbault GG (2002) Direct detection of myoglobin in whole blood using a disposable amperometric immunosensor. Anal Chim Acta 460(2): 141-50.
- Rathee K, Dhull V, Dhull R, Singh S (2016) Biosensors based on electrochemical lactate detection: A comprehensive review. Biochem Biophys reports 5: 35-54.
- Mihailescu CM, Stan D, Iosub R, Moldovan C, Savin M (2015) A sensitive capacitive immunosensor for direct detection of human heart fatty acid-binding protein (h-FABP). Talanta 132: 37-43.
- Karimian N, Vagin M, Zavar MHA, Chamsaz M, Turner APFF, et al. (2013) An ultrasensitive molecularly-imprinted human cardiac troponin sensor. Biosens Bioelectron 50: 492-498.
- Silva BVM, Rodríguez BAG, Sales GF, Sotomayor MDPT, Dutra RF (2016) An ultrasensitive human cardiac troponin T graphene screen-printed electrode based on electropolymerized-molecularly imprinted conducting polymer. Biosens Bioelectron 77: 978-985.
- Zuo J, Zhao X, Ju X, Qiu S, Hu W, et al. (2016) A new molecularly imprinted polymer (MIP)-based electrochemical sensor for monitoring cardiac troponin I (cTnI) in the serum. Electroanalysis 28(9): 2044-2049.
- Moreira FTC, Dutra RAF, Noronha JPC, Sales MGF (2012) Surface imprinting approach on screen printed electrodes coated with carboxylated PVC for myoglobin detection with electrochemical transduction. Procedia Eng 47: 865-868.
- Moreira FTC, Dutra RAF, Noronha JPC, Sales MGF (2013) Electrochemical biosensor based on biomimetic material for myoglobin detection. Electrochim Acta 107: 481-487.
- Phonklam K, Wannapob R, Sriwimol W, Thavarungkul P, Phairatana T (2020) A novel molecularly imprinted polymer PMB/MWCNTS sensor for highly-sensitive cardiac troponin t detection. Sensors Actuators B 308: 127630.
- Regan TMO, Riordan LJO, Pravda M, Sullivan CKO, Guilbault GG (2002) Direct detection of myoglobin in whole blood using a disposable amperometric immunosensor. Analytica Chimica Acta 460(2):141-150.
- Sun L, Li W, Wang M, Ding W, Ji Y (2017) Development of an electrochemical impedance immunosensor for myoglobin determination. Int J Electrochem Sci 12(7): 6170-6179.
- Puri N, Mishra SK, Niazi A, Srivastava AK, Rajesh (2015) Physicochemical characteristics of reduced graphene oxide based pt-nanoparticles-conducting polymer nanocomposite film for immunosensor applications. J Chem Technol Biotechnol 90(9): 1699-1706.
- Wang Y, Han M, Ye X, Wu K, Wu T, et al. (2017) Voltammetric myoglobin sensor based on a glassy carbon electrode modified with a composite film consisting of carbon nanotubes and a molecularly imprinted polymerized ionic liquid. Microchim Acta 184(1): 195-202.
- Moreira FTC, Sharma S, Dutra RAF, Noronha JPC, Cass AEG, et al. (2015) Detection of cardiac biomarker proteins using a disposable based on a molecularly imprinted polymer grafted onto graphite. Microchimica Acta 975-983.
- Karimi M, Rabiee M, Tahriri M, Salarian R, Tayebi L (2019) A graphene based-biomimetic molecularly imprinted polyaniline sensor for ultrasensitive detection of human cardiac troponin T (cTnT). Synth Met 256: 116136.
- Landim VPA, Silva BVM, Sobral Filho DC, Dutra RF (2021) A novel redox‐free immunosensor concept based on cobalt phthalocyanine@carbon nanotubes pseudocapacitor for cardiac B‐type natriuretic peptide detection. Electroanalysis 33(11): 2302-2309.
- Li X, Liu L, Dong X, Zhao G, Li Y, et al. (2019) Dual mode competitive electrochemical immunoassay for B-type natriuretic peptide based on GS/SnO2/polyaniline-Au and ZnCo2O4/N-CNTs. Biosens Bioelectron 126: 448-454.
- Karaman C, Karaman O, Atar N, Yola ML (2021) Electrochemical immunosensor development based on core-shell high-crystalline graphitic carbon nitride@carbon dots and Cd5Zn0.5S/d-Ti3C2Tx MXmxene composite for heart-type fatty acid-binding protein detection. Microchim Acta 188(6): 182
- Feng YG, Zhu JH, Wang AJ, Mei LP, Luo X, et al. (2021) AuPt nanocrystals/polydopamine supported on open-pored hollow carbon nanospheres for a dual-signaling electrochemical ratiometric immunosensor towards h-FABP detection. Sensors Actuators B Chem 346: 130501.
- O’Regan TM, Pravda M, Sullivan CKO, Guilbault G (2002) Development of a disposable immunosensor for the detection of human heart fatty-acid binding protein in human whole blood using screen-printed carbon electrodes. Talanta 57(3): 501-510.
- Stan D, Mihailescu CM, Iosub R, Moldovan C, Savin M, et al. (2012) Electrochemical studies of homogeneous self-assembled monolayers versus mixed self-assembled monolayers on gold electrode for “label free” detection of heart fatty acid binding protein. Thin Solid Films 526: 143-149.
- Hartati YW, Nurmalasari R, Gaffar S, Subroto T (2017) B-Type natriuretic peptide (BNP) detection using electrochemical immunosensor based on sandwich ELISA with horseradish peroxidase-tetramethylbenzidine system. Procedia Technol 27: 149-150.
- Serafín V, Torrente-Rodríguez RM, González-Cortés A, García de Frutos P, Sabaté M, et al. (2018) An electrochemical immunosensor for brain natriuretic peptide prepared with screen-printed carbon electrodes nanostructured with gold nanoparticles grafted through aryl diazonium salt chemistry. Talanta 179: 131-138.
- Lei YM, Xiao MM, Li YT, Xu L, Zhang H, et al. (2017) Detection of heart failure-related biomarker in whole blood with graphene field effect transistor biosensor. Biosens Bioelectron 91: 1-7.
- Zhuo Y, Yi WJ, Lian WB, Yuan R, Chai YQ, et al. (2011) Ultrasensitive electrochemical strategy for nt-proBNP detection with gold nanochains and horseradish peroxidase complex amplification. Biosens Bioelectron 26(5): 2188-2193.
- Felix FS, Angnes L (2018) Electrochemical immunosensors-A powerful tool for analytical applications. Biosens Bioelectron 102: 470-4878.
- Esteban-Fernández de Ávila B, Escamilla-Gómez V, Campuzano S, Pedrero M, Pingarrón JM 2013 (2013) Disposable amperometric magneto immunosensor for the sensitive detection of the cardiac biomarker amino-terminal pro-B-type natriuretic peptide in human serum. Anal Chim Acta784: 18-24.
© 2023 Dennis Shavelson, This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






