- Submissions

Full Text
Significances of Bioengineering & Biosciences
New Record of Stem Anthracnose and Dieback Disease in Jack Fruit Trees (Artocarpus heterophyllus) from Bengaluru, Karnataka, India
Sharun Roy D Souza, Stephen A and Praveen Kumar Nagadesi*
Department of Botany, School of Life Sciences, St. Joseph’s University, India
*Corresponding author: Praveen kumar N, Department of Botany, School of Life Sciences, St. Joseph’s University, India
Submission: August 01, 2022 Published: August 18, 2022

ISSN 2637-8078Volume5 Issue4
Abstract
At this time the whole world food security is considered, so the study of a highly valuable Jackfruit trees showing stem Anthracnose and dieback diseases is a very urgent problem. A survey was done from summer months of 2020 to the summer months of 2022 in Jack fruit trees growing areas of Bengaluru like Arrupe Nivas and Ashirvad campus to identify different fungal diseases and pathogens causing that disease in it. Different stem and leaf samples were collected from study areas for isolation of fungal pathogens by the latest research methods like Blotter and Culture Potato Dextrose Agar (PDA) medium. Arrupe Nivas, jack fruit leaf and stem samples shown the Colletotrichum gloeosporioides (Penz.) Sacc., Lasiodiplodia theobromae (Pat.) Griffon & Maubl., Aspergillus niger Tieghem and Trichoderma sp; whereas Ashirvad campus, Jack fruit leaf and stem samples showed the C. gloeosporioides, Fusarium oxysporun Schlecht. and Epicoccum nigrum Link. The frequently isolated fungal pathogens from Arrupe Nivas jack fruit trees were C. gloeosporioides and L theobromae, whereas the frequently isolated fungal pathogen from Ashirvad campus jackfruit trees was C. gloeosporioides. The pathogenicity test confirms that the anthracnose and dieback diseases are responsible for the death of Jackfruit trees in Arrupe Nivas and dieback disease in Ashirvad campus. The similar type of diseases symptoms was also observed in the Jackfruit growing areas of Bengaluru, Karnataka, India. For the first time the Anthracnose in stem and dieback in trees of jackfruit growing areas of Bengaluru of Karnataka was reported.
Keywords: Anthracnose; Dieback disease; Jack fruit; Colletotrichum gloeosporioides; Lasiodiplodia theobromae
Introduction
Jackfruit is one of the commonly consumed fruits in the tropical regions from the ancient times. Scientifically it is known as A. heterophyllus which is a medium-sized, evergreen tree species, growing up to 8-25m in height, belonging to the family of Moraceae. The tree stem usually grows straight and of diameter 30-80cm [1]. The tree is commonly known as ‘Jackfruit tree’ in Bangladesh-Kathal, in Brazil, Jaqueira, in Germany Jackfruchtbaum, in Indonesia Nangka or Nongko etc. In India, it is called by unique vernacular names like, halasu (Kannada), kathal (Hindi), phanas (Marathi), pila or pilavu or chakka (Tamil & Malayalam) and so on [2]. It is a tropical, climacteric fruit, native to Western Ghats of India, Asia, Africa and some regions of South America.
The bark of the tree is rough, dark grey to brownish colour and scaly. Almost all the living parts of the tree exudes gum-like white latex when injured. The tree leaves are dark green on the adaxial side and dull green on the abaxial side, glabrous, leathery and obovate to elliptical in shape, margin entire, apex is rounded or blunt with a short, pointed tip. The inflorescence is solitary, borne axillary on special lateral shoots. The male flower heads are barrel-shaped, composed of sterile and fertile flowers which are closely embedded on a receptacle. The fruit is spiky, composite and one of the largest edible fruits found in the world. A healthy jackfruit tree can produce more than 200 jackfruits at a time if the environmental conditions found to be favourable [3]. People and livestock depend on Jackfruit which is a non-seasonal fruit-crop and so, it is considered as the ‘poor man’s food’ [4].
India is the largest producer of Jackfruit in the world. India’s Jackfruit production is of 1.4 million tons, whereas Bangladesh produces 926 tons and stands second in the world. It is a national fruit of Bangladesh [5]. Thailand, Indonesia and Nepal are the other top Jackfruit producing countries [6]. According to the National Horticulture board report of India, the area and production of Jackfruit in the year 2017-18 had been 185 ha and 1830 & in the year 2018-19, the area and production had been 187 ha 1815 [7]. Among the highest Jackfruit producing states in India, Tripura stands first. According to the National Horticulture Board (NHB) data 2020, the total cultivation of jackfruit in Tripura has been 291.59 tonnes and it has contributed 16.84% of its share to India. Orissa stands second in the top position by producing 232.79 tonnes which is 13.44% of its share to India. The state government of Kerala has declared ‘Jackfruit’ as the state’s official fruit. In Kerala, the total production of Jackfruit includes 190.14 tonnes, which is 19.98% of its share to India [8]. Even Tamil Nadu has Jackfruit as the official state fruit Panruti district of Tamil Nadu is considered as the ‘Jackfruit paradise’ [9].
The article analyzes many literary data in this area along with benefits of Jackfruit. According to Ranasinghe [1]. A. heterophyllus is a highly beneficial fruit in terms of its nutrition and health benefits. The dietary fibre is rich in jackfruit which helps in maintaining smooth bowel movements, thus preventing constipation problems [1]. The fruit is rich with carbohydrates, proteins and Thiamine; types of amino acids (proteins) in Jackfruitarginine, cystine, leucine, methionine, threonine, tryptophan; Vitamin C and B-complex vitamins (vitamin B6), riboflavin and folic acid, minerals-calcium, potassium iron, magnesium; carotenoids, other secondary metabolites-flavonoids, volatile acid sterols, lignans, isoflavones, saponins and tannins. As the Jackfruit is well known for its rich nutritive value, it is obvious that the fruit also comes with a package of high value of pharmacological properties like, antibacterial, antifungal, antidiabetic, anti-inflammatory and antioxidant properties. The potassium rich fruit pulp aids in reducing the blood pressure and keeps the heart and blood vessels healthy, which prevents the person from having heart attacks [10,11].
The diseases that attack the jackfruit (A. heterophyllus) is various kinds of pathogens, which lowers the crop production drastically. One of the most common diseases that affects Jackfruit tree is Anthracnose in stem, which is caused by C. gloeosporioides, a fungal plant pathogen [12]. It attacks the flowers, leaves, twigs and fruits even in the storage condition. As a result, dark, depressed lesions, brown necrotic areas appear on the leaves and stems. It is pathogenic to more than 470 types of host plants at various stages of developments [13,14]. To name a few plants Prunus dulcis (almond), Persea americana (avocado), Malus domestica (apple), Coffea arabica (coffee), Psidium guajava (guava), Selenicereus undatus (dragon fruit), Fragaria sp. (strawberry) and Carica papaya (papaya) etc. [15-21]. All these are economically important crops. C. gloeosporioides has two important reproductive stages- Teleomorph (Sexual stage) and Anamorph (Asexual stage). In the sexual stage, it is called as Glomerella cingulata and in the asexual stage, C. gloeosporioides [22]. Another most common disease of the jackfruit is dieback disease in trees is caused by a L. theobromae (Syn: Botryodiplodia theobromae). The infected areas appear to be oval shaped, with blackish centres and brownish margins [23]. The literature reveals L. theobromae fungal pathogen causing dieback in various other plant species like cocoa [24], mango [25,26], strawberry [27] etc. The temperature of 25 °C-30 °C and relative humidity of 80-85% favours the development of the dieback disease in plants. Anthracnose in stem and dieback diseases in trees, have been a constant problem for the fruits and vegetable cultivars across the world. It has badly affected the market value in the past and continues to be even now [23]. In addition, the preparation of ecologically clean products of jack fruit is one of the main problems due to diseases in it. So, present study aims at identification of fungal pathogens causing anthracnose in stem and dieback disease in Jackfruit trees growing areas of Bengaluru, Karnataka, India and also aimed at solving the global problem of Jackfruit tree disease.
Materials and Methods
Figure 1: The outline of Bengaluru showing the study area.
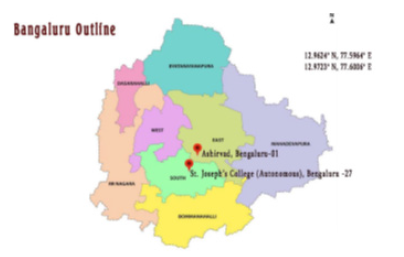
A survey was conducted in the months of late September 2020 to June in the year 2022 in different jackfruit growing areas of Bengaluru like St. Joseph’s College (Autonomous) campus, and Ashirvad campus of Karnataka, India; having longitude and latitude of12.9624°N, 77.5964°E and 12.9723°N, 77.6006°E respectively (Figure 1). It was noted that the unique fungal disease symptoms in A. heterophyllum was observed in many other urban areas of Bengaluru city also. The age of both the A. heterophyllum trees plantation seems to be approximately 15-20 years (Figure 2). Different stem and leaf samples were collected from study sites and is used for isolation of fungal pathogens by the latest research methods like Blotter and Culture Potato Dextrose Agar (PDA) medium.
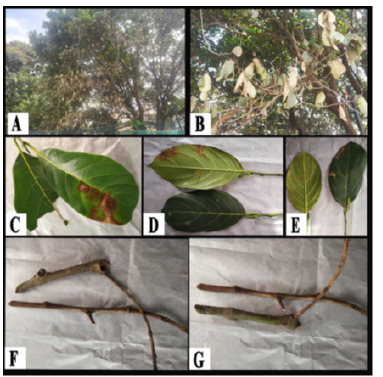
Figure 2:Sample collection from two different Artocarpus heterophyllus trees. (A, C, D, F): Samples of leaf and stem from diseased Artocarpus heterophyllus tree of St. Joseph’s College (Autonomous) campus, Shantinagar, Bengaluru- 27. (B, E, G): Samples of leaf and stem from diseased Artocarpus heterophyllus tree of Ashirvad campus, near St. Mark’s Road, Bengaluru.
Blotter method of isolating fungi
10 samples of infected leaves and stems of the tree were collected from both the study areas the diseased A. heterophyllus trees for isolation of fungal pathogens in it. The twigs samples were cleaned under slow running tap water and air dried on the blotting papers. The symptoms of Anthracnose and dieback disease were noted and photographed. The infected areas on leaf and stem were cut 1mm size bits with the help of scalpel. The surface sterilization of the leaf and stem bits was done by treating them with Sodium hypochlorite (Na2HCl2) and 70% ethanol. The sample bits were kept for 15 minutes and immediately transferred into the distilled water. The Petri plates containing required size of blotting papers is wetted and autoclaved. Under sterile conditions the three bits of leaf and stem were transfer into the Petri plates (3 replicates each) and incubated at 27 OC temperature with 90% relative humidity for seven days. The isolated fungal colonies were sub culture in PDA slants for furthers identification of fungal pathogen [28].
Isolation of fungi by using PDA medium
200g of peeled potato is taken. Cut into small pieces and boiled in 500ml of H2O till it is easily squashed by the glass road. The solution is then filtered using what man no.1 filter paper and poured it into the beaker which contains 20g of dextrose, 15g of Agar with 1000ml of distilled water. It is then stirred well and heated in the micro-Owen until the solution gets transparent. The nutrient media is poured into the 250ml conical flask and corked tightly with the sterile cotton plug. Twelve petri-plates were washed with distilled water and both medium containing conical flasks and petri-plates were autoclaved for the complete sterilization. The laminar air flow is kept ready after sterilizing the floor with 70% ethanol and keeping the UV light on for 15 minutes. The surface sterilization of samples was done as described above Under the sterile conditions the PDA medium was poured into the petri-plate and waited for solidification. After solidification, the infected leaf and stem cut pieces were placed in PDA medium by using the forceps. The petri-plates were categorized into two different sets based on the different diseased tree samples. One of the sets included leaf and stem samples of St. Joseph’s college and the other sets included leaf and stem samples of Ashirvad, Bengaluru. The plates were labelled using the marker. Finally, the petri-plates were kept in the incubation chamber for 7 days under 27±2 °C. The isolated fungal colonies were sub cultured and used for identification of fungal pathogens [29,30].
Identification of the fungi
After placing the petri-plates in the incubation chamber for five days, the fungal growth started appearing. A small portion of each of the fungal colonies grown in the petri-plates taken using the inoculation loop, under sterile conditions and slides were prepared. The fungal sample was teased nicely so that the fungal colonies are not lumped together. Cotton blue stain was used to stain the fungus. The prepared slide was observed in the Onix Vision Trinocular microscope EX30, which has camera and the computer screen attached for the visualization of fungi. The identification of the fungus was done based on the morphological characters of the fungal pathogen with the help of reference manuals. They were compared with the standard works of manual of soil fungi [31], Hyphomycetes [32], A Manual of Penicillium [33], Manual of Aspergillus [34] and Soil fungi [35].
Pathogenicity test
Fresh healthy A. heterophyllus twigs consisting of a minimum number of 15-20 leaves were used for the pathogenicity test. The surface sterilization of the healthy twig was done using Sodium hypochlorite and 70% ethanol in excess to clear the possible contaminants from the healthy twigs. A small portion of the epidermal tissue of the abaxial and adaxial side of the leaves as well as of the bark on stem was removed using a sterilized blade. The isolated fungal pathogen was identified as pathogenic from infected samples was isolated in pure form. The fungal pathogen was applied on the damaged parts of the leaves and the stem of fresh samples. A small piece of sterilized cotton was wetted with the distilled water and placed on the injured parts, so that the pathogens receive necessary moisture for the growth. The twig is then placed carefully inside the polythene cover with moist cotton plug and sealed. The polythene cover is labelled accordingly and placed in the incubation chamber for 7 days, at 27±2 °C temperature. After seven to ten days the symptoms were developed in artificially inoculated fresh samples. From these artificially infected samples the fungal pathogen was re-isolated and identified [30]. This pathogenicity test was used for confirmation of diseases like Anthracnose in stem and Dieback disease in trees of Jack fruit by fungal pathogens isolated.
Result
The utilization of bioplastics for shopping is as of now is extremely normal [3]. After their underlying use they can be reused and afterward biodegraded in soil [30]. Plate and compartments for organic products, vegetables, eggs, meat, bottles for soda pops and dairy items and rankle foils for leafy foods are currently made from bioplastics [30]. In addition, bioplastic is widely used in biomedical field applications and in paper coating by food industries [31].
Figure 3:Potato dextrose media based Fungal culture
plates.
(A, B): Fungal growth from leaf and stem samples of St.
Joseph’s College (Autonomous) campus, Shantinagar,
Bengaluru- 27.
(C, D): Fungal growth from leaf and stem samples of
Ashirvad campus, near St. Mark’s Road, Bengaluru.
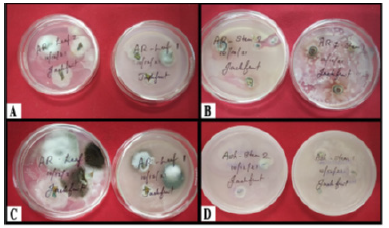
From Arrupe Nivas, the leaf and stem Jackfruit samples, C. gloeosporioides, L. theobromae, A. niger and Trichoderma sp. were isolated. Whereas from Ashirvad campus, the leaf and stem Jackfruit samples, C. gloeosporioides, F. oxysporum and E. nigrum were isolated. The frequently isolated fungal pathogens from Arrupe Nivas jack fruit trees were C. gloeosporioides and L. theobromae; whereas the frequently isolated fungal pathogen from Ashirvad campus jackfruit trees was C. gloeosporioides (Figure 3, 4). The death of Jackfruit trees in Arrupae nivas campus was due to the anthracnose on stem and dieback disease caused by both C. gloeosporioides and L. theobromae respectively. The death of jackfruit trees in Ashirvad campus was due to the dieback disease caused by C. gloeosporioides. The sunken, water-soaked, black to dark brown legions begins to appear on stem and leaf of Jackfruit plants. Conidial masses were produced on the infected stem and leaf during the favourable conditions like the rainy seasons and humid weather. The necrosis of infected stem and leaves appears due to the asexual bodies ‘acervuli’ produced. A small flask shaped structure with a short mass of conidiophores is formed on the surface of necrotic spots on stem and leaves of jackfruit. The conidia escape through an opening of acervuli through necrotic spots. There will be also long brownish coloured projections emerged from acervuli called setae which also comes out through necrotic spots. Based on the above symptoms in infected stem and leaves of jackfruit the disease was identified as anthracnose disease caused by C. gloeosporioides (Figure 2).
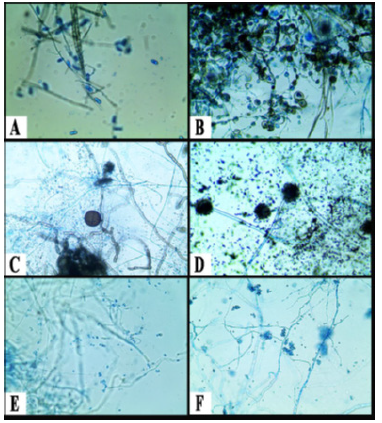
Figure 4:Identification of different fungal pathogens
isolated from fungal pure cultures.
(A): Colletotrichum gloeosporiodes (B): Lasiodiplodia
theobromae (C): Epicoccum nigrum (D): Aspergillus niger
(E): Fusarium oxysporun (F): Trichoderma spp.
The discolouration and darkening of the bark from the tip to downwards was observed in infected jackfruit trees in both the study areas. The young twigs of jack fruit tree start withering from the base and the infected leaves turn brown with their margins rolling upwards. The infected leaves, twigs and branches of jackfruit tree showed necrosis, dried and fall off. In severe condition the branches dry one after another and entire plant died. the fungal pathogen producing pycnidia, two-celled, dark brown, striated conidia in infected parts of Jackfruit trees. These conidia were then dispersed by wind or rain-splash which fall on the damaged parts of the tree and develop disease in health parts of jack fruit. Based on the above symptoms in infected Jackfruit trees the disease was identified as dieback disease caused by L theobromae and C. gloeosporioides (Figure 2). Both the anthracnose in stem and dieback disease in trees are found to cause stunted tree growth and severe decline in fruit crop in A. heterophyllus, which has resulted in significant yield losses to the Jackfruit cultivars.
Pathogenicity test
The healthy A. heterophyllus twigs were artificially induced with the fungal pathogens C. gloeosporioides and L. theobromae to compare and contrast the disease symptoms of the infected healthy twig with that of the diseased tree. After seven to ten days, it was observed that the infection induced healthy twig developed similar symptoms that of anthracnose and dieback disease of Jackfruit (Figure 5). In case of anthracnose disease, the sunken, water-soaked, black to dark brown legions begins to appear on stem and leaf of Jackfruit artificially infected plants whereas in case of dieback disease the fungal pathogen producing necrotic spots on artificially infected leaves and stem parts, and the pathogen producing pycnidia, two-celled, dark brown, striated conidia also. The artificially inoculated twigs were carefully removed from the polythene bags and re-isolation was done. The re-isolated fungi were identified as C. gloeosporioides and L. theobromae so the present pathogenicity study confirms that the anthracnose and dieback diseases are responsible for the death of Jackfruit trees in Arupae nivas and die back disease in Ashirvad campus.
Figure 5:Pathogenicity Tests using the healthy twig of Artocarpus heterophyllus trees. (A & D): Healthy twigs of Artocarpus heterophyllus trees. (B & C): Development of anthracnose and dieback disease after infecting the healthy twigs with the fungal samples of the diseased Artocarpus heterophyllus tree from St. Joseph’s College (Autonomous) campus, Shantinagar, Bengaluru-27. (E & F): Development of anthracnose and dieback disease after infecting the healthy twigs with the fungal samples of the diseased Artocarpus heterophyllus tree from Ashirvad campus, near St. Mark’s Road, Bengaluru.

Discussion
A. heterophyllus is a high value fruit, is also marketed as ‘flagship commodity’, because of its potential to provide sustainable income to the local farming communities. It possesses antibacterial, anti-inflammatory, antidiabetic, antioxidant, antimicrobial and immunomodulatory properties. Consumption of Jackfruit has inevitable positive impact on human health. If such a medically as well as commercially high-valued fruit crop is attacked by the phytopathogens, then there would obviously be huge economical loss for the country [36]. In the present study, the high economic valued jackfruit was found to be affected by anthracnose and dieback diseases in Bengaluru. Borines et al. [36] reports that many other fungal pathogens also are found in the dieback diseased tree of A. heterophyllus like, Fusarium, Pythium and Phytophthora species [36]. The present study confirms that the anthracnose and dieback diseases of A. heterophyllusis due to the fungal pathogens C. gloeosporioides and L. Theobromae respectively. The other fungal species isolated along with these disease-causing pathogens in the Jackfruit tree were namely, Trichoderma sp. F. oxysporum, E. nigrum. and A. niger. C. gloeosporioides was first identified by Peznig. The christening of the pathogen was based on the type of a specimen Vermicularia gloeosporioides which was collected from Citrus in Italy. In India, this phytopathogen was first observed on Coffea arabica and was reported by Butler. McRae identified it as the causal pathogen of anthracnose disease of mango [22]. In the present study, C. gloeosporioides was isolated from leaf and stem samples of Jackfruit, which is responsible for anthracnose disease in Bengaluru.
Bajpai [17], reports the anthracnose disease in the pomaceous fruit, Malus domestica (Apple) affecting the fruit, stolon and crowns. The infection of fungal pathogen was thought to be possible when the fungi enzymatically penetrated the cuticle of the fruit [17]. Coffee is a major commercial export crop and the predominant threat to this cash crop is anthracnose disease. After analysing the isolates of the fungal colonies on coffee reported that 17% of 60 colonies corresponded to the six species of Colletotrichum [18]. Strawberry crop being infected is due to anthracnose fruit rot disease caused by the Colletotrichum sp. C. acutatum seemed to colonize strongly on the foliage parts of these runner plants. The infected runners show lesions on the petiole and roots [19]. Guo et al. [21] reports that in the healthy dragon fruits, small light brown spots started appearing on the surface of the fruits and rapidly developed into sunken soaked lesions having grey to black acervuli of 1cm in diameter [21]. Almonds are commonly affected by anthracnose disease, where the infected fruits are depressed and exhibit round, orange lesions from 5-12mm on the fruit surface [15]. Severely infected fruits become unfit for consumption which results in the dropping of market value for the fruits. Many commercial producers of fruits like Psidium guajava think of giving up cultivation of fruit crop due to the great loss by this disease [20]. In the present study also, the above symptoms were observed in Jackfruit leaf and stem samples, which confirms anthracnose disease is caused by C. gloeosporioides.
The first report of dieback in Strawberry plants in Kenya reveals that the symptoms of dieback began on runners, causing black rot on roots and necrotic discolouration in the crown of the plants [27]. In Theobroma cocoa of Malasia, the isolates of L. theobromae were responsible to cause leaf blight, stem canker and pod rot [24]. Shahbaz [26] found that L. theobromae was associated with the decline of mango trees. As an aggressive and vigorous pathogen, it caused tip dieback and twig blight in mango crops [26] Khanzada et al., denotes referring to the survey done on the infected fields of mango groove that twigs die from the tips becoming black and wood forming into a scorched appearance. Leaves on the infected branch turned brown with margins rolled upwards and fall of leaving the dead branches [25]. In the present study also, the above symptoms were shown in roots, stem, branches and leaves of Jackfruit tree which is infected by L. theobromae. So, in the Jackfruit growing areas of Bengaluru all the trees in orchards were dying due to dieback disease.
Conclusion
Jackfruit is a highly valuable fruit, because of its nutrition and health benefits. A study was done in Jack fruit trees growing areas of Bengaluru like Arrupe Nivas and Ashirvad campus from different seasons of 2020 to the summer months of 2022 to identify different fungal diseases and pathogens causing diseases in it. The Blotter and culture PDA medium methods shown the fungal colonies from Arrupe Nivas, jack fruit leaf and stem samples which were identified as C. gloeosporioides, L. theobromae, A. niger and Trichoderma sp; whereas Ashirvad campus, Jack fruit leaf and stem samples showed the occurrence of C. gloeosporioides, F. oxysporun and E. nigrum. The frequently isolated fungal pathogens from Arrupe Nivas jack fruit trees was C. gloeosporioides and L. theobromae; whereas from Ashirvad campus jackfruit trees was C. gloeosporioides Based on the symptoms in infected stem and leaves of jackfruit the disease was identified as anthracnose disease caused by C. gloeosporioides and dieback disease caused by L theobromae in case of Arrupe Nivas jackfruit trees whereas dieback disease caused by C. gloeosporioides in case of Ashirvad campus jackfruit trees. The pathogenicity test also confirms that the anthracnose and dieback diseases are responsible for the death of Jackfruit trees in Arrupe Nivas and dieback disease in Ashirvad campus. The similar fungal disease symptoms were observed in all the jackfruit growing orchards of urban Bengaluru city, Karnataka. For the first time the Anthracnose in stem and dieback in trees of jackfruit growing areas of Bengaluru of Karnataka was reported. The further research is required to control both the diseases to safe guard the Jackfruit trees.
Acknowledgement
The authors are thankful to Fr. Swebert D’Silva SJ, the Pro- Chancellor and Fr. Victor Lobo SJ, the Vice-Chancellor of St. Joseph’s College (Autonomous), and Fr. Arun Louis SJ, the Superior of Ashirvad, Bengaluru, for permitting to collect the diseased tree samples. The authors are also thankful to Fr. S. Xavier SJ, The Research Director, Dr. Jacob Paul, HOD and Dr. Vaishnavi, PG Coordinator, the Faculty, Mr. Sagainathan, Lab assistant, Department of Botany for the whole hearted support in the research work. A special thanks to Fr. Denzil.
References
- Ranasinghe RASN, Maduwanthi SD, Marapana RAUJ (2019) Nutritional and health benefits of jackfruit (Artocarpus heterophyllus): a review. Int J Food Sci, pp. 1-12.
- Cabi (2019) Invasive species compendium. Detailed coverage of invasive species threatening livelihoods and the environment worldwide. Datasheet, Cherax Quadricarinatus (Redclaw Crayfish) Cabi, Wallingford, United Kingdom.
- Saha S, Sarker M, Haque AR, Nayeem TA, Maukeeb AR (2022) A review on tropical fruit: jackfruit (Artocarpus heterophyllus). Asian Journal of Advances in Research 13(2): 25-34.
- Afroz M, Rahman MA (2016) Survey on the diseases of jackfruit and some aspects of control measures for gummosis disease in bangladesh. Eco-Friendly 9(2): 10-14.
- Khan AU, Khan AU, (2020) Infested and healthy plant and fruit of jackfruit: insect pests and diseases of jackfruit plant and fruit: a pictorial study. Research Gate, pp. 1-23.
- Ullah Khan A, Choudhury AR, Maleque A, Dash CK, Talucder SA, et al. (2021) Management of insect pests and diseases of jackfruit (Artocarpus heterophyllus) in agroforestry system: a review. Acta Entomology and Zoology 2(1): 37-46.
- (2019) Area and production of horticulture crops for 2018-19 (3rd advance estimates). National Horticulture Board.
- (2020) Top 10 Jackfruit producing states in India [states with highest jack fruit productions]. The Indian Blog.
- Prasad S (2022) Panruti: the jackfruit paradise. The Hindu.
- Prakash Om, Rajesh K, Anurag M, Rajiv G (2009) Review article Artocarpus heterophyllus (jackfruit): an overview. Pharmacognosy Reviews 3 (6): 353-358.
- Tulyathan V, Tananuwong K, Songjinda P, Jaiboon N (2002) Some physicochemical properties of jackfruit (Artocarpus heterophyllus Lam) seed flour and starch. Science Asia 28: 37-41.
- Srivastava MP, Mehra R (2006) Diseases of minor tropical and sub-tropical fruits and their management. Diseases of Fruits and Vegetables: Volume II, pp. 559-632.
- Pavitra K, Rakesh RS (2017) Characterization of colletotrichum species responsible for anthracnose diseases of various fruits. International Journal of Pure & Applied Bioscience 5 (1): 48-56.
- Nelson SC (2010) Mango anthracnose (Colletotrichum gloeosporiodes).
- Lópes-Moral A, Agustí-Brisach C, Lovera M, Arquero O, Trapero A (2020) Almond anthracnose: current knowledge and future perspectives. Plants 9(8): 945.
- Kimaru KS, Muchemi KP, Mwangi JW (2020) Effects of anthracnose disease on avocado production in Kenya. Cogent Food and Agriculture 6(1).
- Bajpai VK, Choi SW, Cho MS, Chul S (2009) Isolation and morphological identification of apple anthracnose fungus of colletotrichum sp. kv-21. Korean Journal of Environmental Agriculture 28: 442-446.
- Cristóbal Martínez AL, Yáñez Morales MDJ, Vidal RS, León OS, Hernández Anguiano AM (2017) Diversity of Colletotrichum species in coffee (Coffea arabica) plantations in Mexico. European Journal of Plant Pathology 147: 605-614.
- Mertely JC, Peres NA (1969) Anthracnose fruit rot of strawberry, p. 207.
- Ansari TH, Yoshida T, Meah MB (2000) An Integrated approach to control anthracnose of guava (Psidium Guajava). Pakistan Journal of Biological Sciences 3(5): 791-794.
- Guo LW, Wu XY, Ho HH, Su YY, Mao ZC, et al. (2014) First report of dragon fruit (Hylocereus undatus) anthracnose caused by Colletotrichum truncatum in China. Journal of Phytopathology 162(4): 272-275.
- Pavitra K, Rajender S (2017) Anthracnose of mango incited by Colletotrichum gloeosporioides: a comprehensive review. International Journal of Pure & Applied Bioscience 5(1): 48-56.
- Adikaram N, Manawadu L, Jayasinghe L, Yakandawala D (2020) First report of Lasiodiplodia theobromae rot in ripe jack fruit (Artocarpus Heterophyllus Lam.) in Sri Lanka. Indian Phytopathology 73: 583-585.
- Huda-Shakirah AR, Nor NMIM, Zakaria L, Hawa Mohd M (2022) Lasiodiplodia theobromae as a causal pathogen of leaf blight, stem canker, and pod rot of theobroma cacao iln Malaysia. Scientific Reports 12(1): 1-14.
- Khanzada MA, Lodhi AM, Lodhi, Saleem S (2004) Pathogenicity of Lasiodiplodia theobromae and fusarium solani on mango. Pakistan Journal of Botany 36(1): 181-189.
- Shahbaz M, Iqbal Z, Saleem A, Anjum MA (2009) Association of Lasiodiplodia theobromae with different decline disorders in mango (Mangifera indica Lam). Pakistan Journal of Botany 41(1): 359-368.
- Nam YH, Park YS, Kim HS, Kim TI, Lee EM, et al. (2016) First report of dieback caused by Lasiodiplodia theobromae in strawberry plants in Korea. Mycobiology 44(4): 319-24.
- Muhammad I, Noreen M, Maqbool MM, Hussain T, Azam S (2015) Analysis of fungal diversity impacts on pinus roxburghii seeds from pine forest and plant nurseries of Azad Kashmir, pakistan. Pakistan Journal of Botany 47(4): 1407-1414.
- Tharmila S, Jeyaseelan EC, Thavaranjit AC (2011) Preliminary screening of alternative culture media for the growth of some selected fungi. Archives of Applied Science Research 3(3): 389-393.
- Aneja KR (2003) Experiments in microbiology, plant pathology and biotechnology. New Age International Pvt Ltd Publishers, New Delhi, India, p. 632.
- Gilman JC (1957) A Manual of Soil Fungi. Science, New Delhi, India, 126(3265): 173-174.
- Subramanian CV (1971) Hyphomycetes. An account of Indian species, except Cercosporae. Counc Agri Res, New Delhi, India.
- Raper KB, Thom C (1949) A manual of penicillium. Baltimore: Williams and Wilkins Co, USA.
- Raper KB, Fennell DI (1965) The genus aspergillus. Baltimore: Williams and Wilkins Co, USA.
- Domsch KH, Gams W, Anderson TH (1980) Compendium of Soil Fungi. Academic Press, New York, USA.
- Borines LM, Palermo VG, Guadalquiver GA, Dwyer C, Drenth A, et al. (2014). Jackfruit decline caused by Phytophthora palmivora (butler). Australasian Plant Pathology 43: 123-129.
© 2022 © Praveen Kumar Nagadesi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






