- Submissions

Full Text
Significances of Bioengineering & Biosciences
Nickel-Modified Carbon Paste Electrode for the Methanol Fuel Cell
Raja Maallah* and Abdelilah Chtaini
Beni Mellal Faculty of Science and Technology, Molecular Electrochemistry and Inorganic Materials Team, Sultan Moulay Slimane University, Morocco
*Corresponding author: Raja Maallah, Beni Mellal Faculty of Science and Technology, Molecular Electrochemistry and Inorganic Materials Team, Sultan Moulay Slimane University, Morocco
Submission: August 11, 2021 Published: September 24, 2021

ISSN 2637-8078Volume5 Issue3
Abstract
This work aims to study the electro-oxidation of methanol, in an alkaline solution at a nickelmodified carbon paste electrode Ni -CPE, by cyclic voltammetry, impedance measurements and chronoemperometry. X-ray carried out morphological characterizations by and Microscopy Electronic Transmission (MET). Ni has been found to have a better catalytic activity for methanol oxidation; they are simple, less expensive and stable.
Keywords: Methanol fuel cell; Electro catalysis; Modified electrodes; Nickel catalyst; Chronoamperometry ; Impedance spectroscopy
Abbreviations: MET: Microscopy Electronic Transmission; DMFC: Direct Methanol Fuel Cell; PEM: Proton Exchange Membrane; SCE: Calomel Electrode Saturated; Ni-CPE: Nickel-Modified Carbon Paste; CPE: Carbon Paste Electrode; ET: Electrode
Introduction
Fuel cells have been identified as a promising energy source for portable electronic devices and transport because they convert the chemical energy of a fuel for electrical energy; with low emissions and no moving parts. The Direct Methanol Fuel Cell (DMFC) is an electrochemical device that converts energy by chemical reactions (electro-oxidation of ethanol) into electrical energy. The high demand for energy, coupled with concerns about environmental pollution and rising costs of fossil fuels have created a great need for clean and efficient energy sources [1]. Direct methanol fuel cells and direct ethanol fuel cells have been projected to be good candidates for filling gaps with advanced batteries to power mobile and portable electronic devices due to their exceptionally high specific energy [2]. Methanol has been considered as an electrical energy source for the fuel cell, because it can be transformed into a hydrogen rich fuel gas in a fair, simple and efficient way, by steam or automatic thermal reforming [3,4]. Methanol is a liquid fuel that is easy to transport, and store compared to hydrogen gas. On this basis, Direct Methanol Fuel Cells (DMFC) appear to be more attractive technologies than Direct Ethanol Fuel Cells (DEFC) [5,6]. The performance of Direct Methanol Fuel Cells (DMFC) is further limited by the electrocatalysts available for ethanol oxidation, which are mainly based on expensive noble metals such as platinum or its alloys [7]. Previous research on the development of DMFC has focused mainly on DMFCs called PEM (Proton Exchange Membrane) which use PEM as electrolyte, a catalyst based on Pt (platinum) on the anode, and a pure Pt catalyst on the cathode [8,9]. In this work, the Ni-CPE anodes are respectively prepared by the electrodeposition of the layer of nickel-based catalyst on the surface of the carbon paste. The morphology and structure of the relative activity of the Ni- CPE anode were evaluated by the MET. The relative activities of the prepared electrodes were tested for the methanol oxidation reaction.
Experimental Part
Apparatus
Electrochemical experiments were performed using a Volta Lab potentiostat (model of PGSTAT 100, Eco Chemie BV, Utrecht, Netherlands) controlled by the software data processing of general purpose electrochemical systems (Volta Lab software Master 4). All electrochemical experiments were carried out in a compartment of a standard three-electrode cell. The reference electrode is SCE (Calomel Electrode Saturated) and the counter electrode is platinum. All electrode potentials have been referred to the reference electrode. The carbon paste electrode modified by Nickel (Ni-CPE), were used as the working Electrode (ET) [10,11].
Reagents and solutions
All the chemicals used in this study are of good quality. Powder graphite (RWB spectroscopic grade, Ringsdorff-Werke GmbH, Bonn-Bad Godesberg, Germany) was obtained from Aldrich and was used without purification. NiSO4 was obtained from Merck chemicals. The electrolytic solution is the 0.1M KOH. Demineralized water was used throughout this work [12].
Working electrode
The Ni-CPE modified electrode was prepared by manually mixing the nickel compound and the graphite powder by adding paraffin oil at different Ni/CP ratios of 5, 10, 25, 40, 50, 65 and 80% by weight (w/w) to give appropriate ratios (w/w). Ethanol was added to the mixture in a suitable amount as an inert volatile solvent and the resulting mixture was well homogenized by thoroughly mixing the two materials, then this mixture was left in the air for evaporation of the solvent [13]. The composite material obtained (paste) was housed in a cylindrical PTFE tube (the geometric surface of the working electrode is 0.1256cm2). The electrical contact was fitted with a carbon bar.
CPE test for methanol oxidation
(Figure 1) shows the evolution of the recorded methanol oxidation current densities, respectively, on the CPE electrode (curve a) in the absence and in the presence of methanol. We find that the carbon paste electrode shows no activity with respect to the oxidation of methanol.
Effect of the amount of mixed Ni
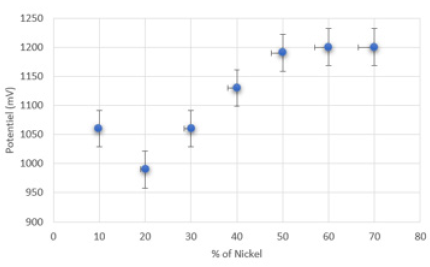
The influence of the percentage of nickel content in the mixed carbon paste was studied by cyclic voltammetry of 0.4mol/ l of methanol in an alkaline solution medium. The voltammetric VC response of the electrode modified by Ni for a different percentage of nickel in the carbon mixture (10%, 20%, 40%, 50%, 60% and 70%) is illustrated in (Figure 2). the electrode modified by 50% (w/w) of Ni in the pulp exhibits remarkably high current densities and the improvement in the onset of fuel oxidation, the value of which decreases considerably. After these results, an amount of 20% Ni (w/w) by weight in the composition of the carbon paste was chosen as the best for other experiments.
Figure 1: Cyclic voltammograms of CPE, illustrating: a-oxidation of methanol, b-without methanol, in an alkaline medium.
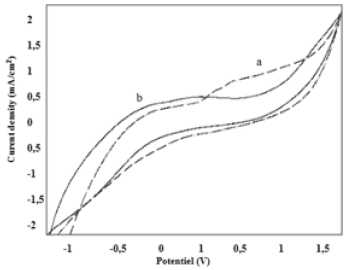
Figure 2: Graph of the Ni content in the carbon paste mixture Percentage (20/80) of the VC in alkaline medium of concentration 0.4molL-1 of methanol.
Result and Discussion
Characterization of the surface of Ni-CPE
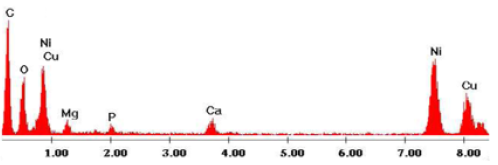
The morphological study of the modified surface was carried out by X-ray and Microscopy Electronic Transmission (MET). The results are illustrated in (Figures 3 & 4), we can note on the photograph I that the surface shows the surface of the unmodified electrode, and the photograph II consists of two layers, the base layer (I) corresponds to the surface of the carbon paste, while the surface layer should correspond to the nickel clusters deposited on the carbon (II). Ni’s film is continuous and homogeneous. From this analysis, it is clear that the electrode was well modified by Ni. The EDX spectrum shows well, intense peaks of Ni.
Figure 3: Characterization of the surface of Ni-CPE.
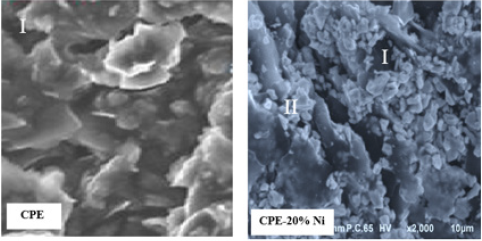
Figure 4: Analysis of 20% Ni-CPE EDX spectra of electron micrographs scanning of the cross section of the Ni-CPE anode: II: Ni and I: CPE catalyst.
Electrochemical characterization of the prepared electrode: Ni-CPE
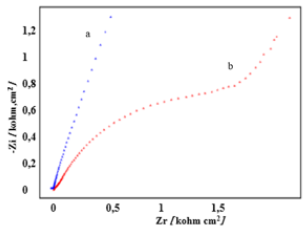
Cyclic voltammograms recorded for the two electrodes: Carbon Paste (CPE) and Nickel-Modified Carbon Paste (Ni-CPE) in an electrolytic medium KOH 0, 1M, have different looks, which means that the Carbon Paste Electrode (CPE) has been well modified by nickel (Figure 5) [14]. The impedance diagrams (EIS) plotted, on the Nyquist plan, respectively for the CPE and Ni-CPE electrodes are illustrated in (Figure 6) [15]. The EIS, corresponding to the electrode Ni-CPE (curve b), in the shape of a semicircle, attributed to the exchange of electrons at the metal/solution interface, on the other hand the EIS recorded for the unmodified electrode (curve a), the points are scattered, and the circle has difficulty closing.
Figure 5: Cyclic voltammograms recorded by: carbon paste electrode and carbon paste electrode modified by Ni, in the 0.1M KOH solution at 100mV/s.
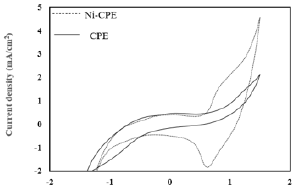
Figure 6: Impedance diagrams recorded in an alkaline medium, for electrodes, a- CPE and b- Ni-CPE.
Oxidation of methanol with the Ni-CPE Electrode
The activity of the prepared electrode (Ni-CPE), was invested by chronoamperometry, on the (Figure 7), we present a comparison between the CPE and Ni-CPE electrodes, opposite, of oxidation of methanol. We can see that the CPE electrode has a low activity for fuel oxidation compared to the Ni-CPE electrode, which shows that the nickel adds value to carbon paste. This comparison was confirmed by cyclic voltammetry (Figure 8), the modified electrode has remarkably high current densities and the onset of oxidation of the fuel takes place around, 1050mV instead of 1450mV for without combustible. The voltammogram unregistered in solution present of combustible shows the existence of two anodic peaks at potential values of about 800 and 600mV. The first one can be attributed to the oxidation of nickel, and the second one, associated to the cathodic peak, corresponds to reversible Ni(OH)2/NiOOH transformation.
Concentration effect
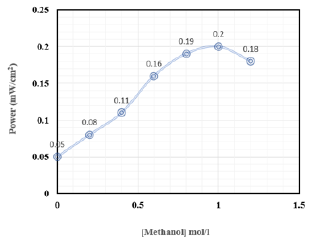
The impedance diagrams, (Figure 9), saved for the electrode Ni- CPE, in an alkaline medium. We find that the semicircles recorded tend to be close as the methanol concentration increases. These semicircles are associated with the electron exchange that takes place during the fuel oxidation reaction. The concentration of the fuel has a positive effect on the performance of the cell studied (Figure 10), a rise in concentration probably leads to an increase in the turnover of the electrode. The number of active sites available is probably very large. The value of the onset decreases which reinforces the chances of the electrode prepared to play the driving role in the fuel cell. The estimated electrical power for the fuel cell studied, makes it possible to study its performances (Figure 11). We find that this power varies depending on the methanol concentration, it has a maximum at a methanol concentration of the order of 1mol/L.
Figure 7: Chronoamperometry curves recorded by: carbon paste electrode and carbon paste electrode modified by Ni.

Figure 9: Impedance diagrams recorded for the Ni-CPE electrode, effect of the variation of the concentration of methanol (0.2 to 1Mol/L).
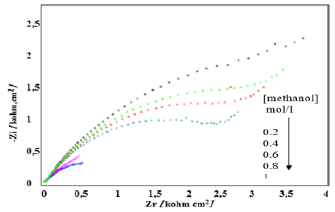
Figure 10: Cyclic voltammograms, illustrating the oxidation of methanol on the Ni-CPE electrode. Effect of methanol concentration (0.2 to 1Mol/L).
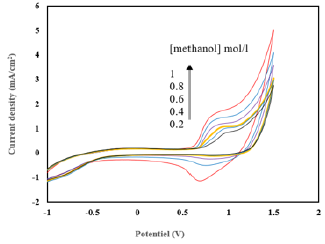
Figure 11: Evolution of electrical power with the concentration of methanol.
Conclusion
Methanol oxidation was carried out by cyclic voltammetry, impedance spectroscopy and chronoamperometry. The modification of mass electrode, Ni improves the current densities of methanol because of its surface oxidation properties, which seems to favor the anodic reaction. The presence of Ni on the graphite carbon matrix has made it possible to generate very high current densities and acceptable electrical powers. The porosity of the Ni- CPE electrode surface facilitates the attachment of the electro active molecule and subsequently its oxidation. No Electrode poisoning was observed during fuel oxidation, the current densities increase with the concentration of methanol.
References
- Wenxin Du, Kayla EM, Daniel FM, Aaron DN, Dong SS, et al. (2012) Palladium–tin alloyed catalysts for the ethanol oxidation reaction in an alkaline medium. ACS Catalysis 2(2): 287-297.
- Shen SY, Zhao TS, Xu JB, Li YS (2010) Synthesis of PdNi catalysts for the oxidation of ethanol in alkaline direct ethanol fuel cells. Journal of Power Sources 195(4): 1001-1006.
- Zhou WJ, Zhou B, Li WZ, Zhou ZH, Song SQ, et al. (2004) Performance comparison of low-temperature direct alcohol fuel cells with different anode catalysts. Journal of Power Sources 126(1-2): 16-22.
- Lamy C, Alexandre L, Véronique LR, Fabien D, Christophe C, et al. (2002) Recent advances in the development of direct alcohol fuel cells (DAFC). Journal of Power Sources 105(2): 283-296.
- Achmad F, Kamarudin SK, Daud WRW, Majlan EH (2011) Passive direct methanol fuel cells for portable electronic devices. Economic Literature 88(5): 1681-1689.
- Faghri A, Zhen G (2008) An innovative passive DMFC technology. Applied Thermal Engineering 28(13): 1614-1622.
- Kwong-Yu C, Jie D, Jiawen R, Shaoan C, Kwok YT (2004) Supported mixed metal nanoparticles as electrocatalysts in low temperature fuel cells. Journal of Materials Chemistry 14(4): 505-516.
- Siné G, Smida D, Limat M, Fóti G, Comninellis C (2007) Microemulsion synthesized Pt∕ Ru∕ Sn nanoparticles on BDD for alcohol electro-oxidation. Journal of the Electrochemical Society 154(2): B170-B174.
- Antolini E (2007) Catalysts for direct ethanol fuel cells. Journal of Power Sources 170(1): 1-12.
- Fujiwara N, Friedrich KA, Stimming U (1999) Ethanol oxidation on PtRu electrodes studied by differential electrochemical mass spectrometry. Journal of Electroanalytical Chemistry 472(2): 120-125.
- Maallah R, Chtaini A (2019) Biomaterials electrodes for degradation of phenol. Anal Bioanal Electrochem 11(9): 1206-1216.
- Sanae D, Harouna M, Abdelilah C (2010) Phosphate modified copper electrodes for methanol fuel cell. Portugaliae Electrochimica Acta 28(4): 241-252.
- Smaini MA, Maallah R, Touzara S, Laghlimi C, Qouatli S, et al. (2017) Electrochemical detection and removal of mercury (II) at DNA modified carbon paste electrode. Biol Syst Open Access 6(1): 1-4.
- Mhammedi MA, Achak M, Chtaini A (2017) Ca10(PO4)6(OH)2-modified carbon-paste electrode for the determination of trace lead (II) by square-wave voltammetry. Journal of Hazardous Materials 161(1): 55-61.
- Laghlimi C, Smaini MA, Maallah R, Touzara S, Qouatli S, et al. (2017) Organic sensor for the detection of ammonium. J Biosens Bioelectron 8(1): 1-3.
© 2021 © Raja Maallah. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






