- Submissions

Full Text
Significances of Bioengineering & Biosciences
Synthesis, Characterization and Antifungal Activities, Aspergillus Niger, Aspergillus Flavus, Candida Albicans of Novel Schiff Base and Schiff Based Resin
Nasira LR1*, Akhtar MM1, Aamna B2 and Mohammed KK3
1M A Kazi Institute of Chemistry, University of Sindh, Pakistan
2Department of Physics Engineering, Istanbul Technical University, Turkey
3H E J Research Institute of Chemistry, International Centre for Chemical and Biological Sciences, University of Karachi, Pakistan
*Corresponding author: Nasira Liaquat Rajput, M A Kazi Institute of Chemistry, University of Sindh, Jamshoro-76080, Pakistan
Submission: July 21, 2021 Published: September 20, 2021

ISSN 2637-8078Volume5 Issue3
Abstract
This work studies the synthesis of a novel Schiff base bis (2- methoxy-1-napthaldehyde) hydrazine hydrate and Schiff based resin poly methylene bis (2- methoxy-1-napthaldehyde) hydrazine hydride via-condensation procedure using with formaldehyde (HCHO) at temperature 70 to 80 °C for 3-4 hours. The synthesized Schiff base BMNHH and resin PMBMNHH were recrystallized from methanol resulting pure compounds. The synthesized compounds were characterized by (CHN), (FT-IR) Thermo Gravimetric Analysis (TGA), EDS analysis and Scanning Electron Microscopy (SEM), Powder X-Ray Diffraction Analysis (XRD). The all results of analytical techniques successfully approved the structure and morphology of newly prepared Schiff base and Schiff based resin. After the characterization the synthesized compounds BMNHH and PMBMNHH was screened against Gram-negative antifungal strains such as, Aspergillus Niger (Gram-negative) Aspergillus flavus (Gram-negative), Candida albicans (Gram-negative) through Agar well diffusion method. The found results conveyed that the tested compounds indicated good sensitivity and enhanced activity against Gram-negative antifungal strains [1,2].
Keywords: Synthesis; Antifungal; Spectroscopy; Condensation; Morphology; Candida albicans
Abbreviations: TGA: Thermo Gravimetric Analysis; SEM: Scanning Electron Microscopy; XRD: X-Ray Diffraction Analysis; FT-IR: Fourier Transform Infrared Spectroscopy; TLC: Thin-Layer Chromatography; TGA: Thermogravimetric Analysis; PDA: Potato Dextrose Agar
Introduction
Schiff base resins are mostly insoluble material and holds poor solubility in organic solvents and sharp melting point [3,4]. Schiff bases resins are play an important role for clinical, analytical and biological applications [5]. Schiff bases are biological active compounds they possesses as lot of such biological activities the organic chemists got interested to synthesize Schiff base [6]. Widely used of antibacterial drugs against bacterial infections had led to severe health problems and infections produced by multidrug-resistant gram-positive pathogens, such as staphylococcus class, which has become a severe problem in hospitals and wide-ranging spectrum antibacterial agents has discovered and modified to number of chemical agent and antibiotics or antibiotics with an effective, wide therapeutic window having broad spectrum activity, and novel mode of action has been taken [7]. Schiff base ligands used for extensive application in the field of catalysis, antimicrobial, anti-tuberculosis and anti-tumor activity [8]. The Schiff base’s azomethine group is an important for biological activities. In addition, it has been showed that a large number of Schiff base complexes such as palladium and platinum have antibacterial, anti-inflammatory, antiviral and antimicrobial activities [9].
Synthesis, characterization of Schiff bases have remained studied is proved that C=N linkage in Schiff bases is an essential part for bioactivity [10]. Schiff bases are significant in many fields, and great assortment in their varied biological activities [11-13]. Their role as antimicrobials, and other biological activities like anticancer, antioxidant, anti-tumor and herbicidal properties from class of penicillin and cephalosporin activities are studied f reported fungal and bacterial strains that have developed resistance to coextensively [14-16]. The Schiff based resins formed important forms of substances and number of antibiotics has intensified the search for novel antibacterial compounds [17,18]. Schiff bases have been formed and showed promising activity against bacteria and other microorganism; chemists are interested to study Schiff bases and use in proposal of medicinal compounds [19]. Schiff base resins have applicability in number of fields of industrial and biological sciences and in synthesis of drugs. They are appropriate in the field of biology, as anticancer, antioxidant, anti-inflammatory, antibacterial, antifungal antiviral and anti-malarial [20,21].
The present work reports the synthetic method for preparing Schiff base (BMNHH) and its resin (PMBMNHH). The newly formed compounds were characterized through different analytical techniques such as, Fourier transform infrared spectroscopy (FTIR) and (CHN), (TGA), EDS analysis, X-ray diffraction analysis (XRD), scanning electron microscopy (SEM). All the calculated values of (CHN, EDS, SEM and XRD) confirmed the crystallographic evaluation, morphology and structure of compounds. After the characterization synthesized compounds successfully applied for antifungal strains such as, Aspergillus Niger (Gram-negative) Aspergillus flavus (Gram-negative), Candida albicans (Gramnegative) by Agar well diffusion method (Figure 1).
Figure 1: Graphical abstract of synthesized compounds.
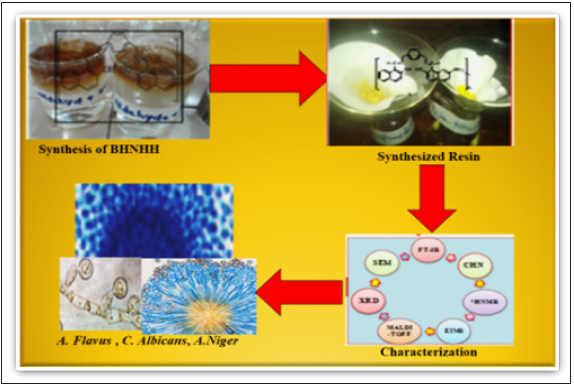
Material and Method
Measurements
Thin-Layer Chromatography (TLC) were observed on precoated silica gel aluminum plates (Kieselgel,60,254, E. Merck, Germany). Visualization of TLC chromatograms were complete at wavelengths of 254 to 365nm. The FT-IR spectra were recorded on thermo Nicolet Corporation, SF-AV 400 USA.SEM analyses were recorded on model-JSM-6380Iv and XRD analysis were recorded D 8-Bruker Japan.
Reagents
All the starting material 2-methoxy-1-naphthaldehyde and hydrazine hydride was purchased from (alfa aesar, Germany), were recrystallized from methanol. Other chemicals such as acetic acid, formaldehyde, methanol, ethyl acetate, n-hexane, NaOH were obtained from E Merck, Germany, solvents and Petri plates added reagents were distinguished conferring to the standard method.
Chemistry
The synthesis of resin included two steps, the first step comprises of a Schiff base BMNHH followed by the preparation of its resin PMBMNHH by common method. The synthetic compounds were then characterized by instrumental techniques such as, CHN elemental microanalysis, Thermo Gravimetric Analysis (TGA), EDS analysis, XRD, and SEM analysis. The represents the formation of Schiff base and its resin respectively. The Schiff base (BMNHH) and resin (PMBMNHH) was screened against the antifungal strains such as, Aspergillus Niger (Gram-negative) Aspergillus flavus (Gramnegative), Candida albicans (Gram-negative) by Agar well diffusion method.
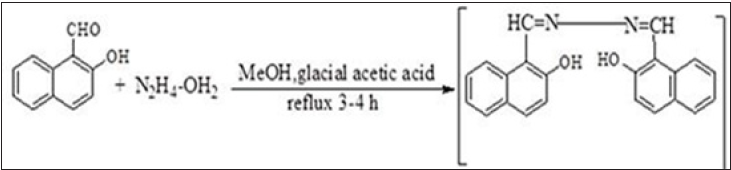
Synthesis procedure of schiff base BMNHH
In a reaction flask 2-methoxy-1-napthalehyde (0.341g, 2mmol) was dissolved in ethanol, 5 to 6 drops of glacial acetic acid were added, hydrazine hydrate (0.03mL, 1mmol) was added in reaction flask and reaction mixture was then refluxed for 3-4 hours. The reaction was observed through TLC. The formation of yellow precipitates indicated the completion of reaction; the precipitates were then filtered washed with n-hexane and dried to become pure product (Figure 2).
Figure 2: Reaction scheme of schiff base BHNHH.
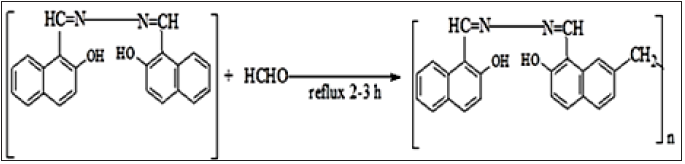
Synthesis procedure of resin PMBMNHH
Schiff base BMNHH (1g) was dissolved in 25ml of distilled water, stirred for 10-15 minutes and 15 drops of 2 molar NaOH was then added. The reaction mixture was refluxed at 60 °C for five minutes, a solution of 37% HCHO was then added. The reaction was refluxed on oil bath at temperature 120 °C for three hour. The precipitates obtained were filtered washed with diethyl ether and n-hexane. The recovered precipitates were then dried in an oven for 1-2 hours (Figure 3).
Figure 3: Reaction scheme of schiff based resin PMBHNHH.
Result and Discussion
Spectral characterization of synthesized compound BMNHH and PMBMNHH infrared spectral studies of synthesized compounds
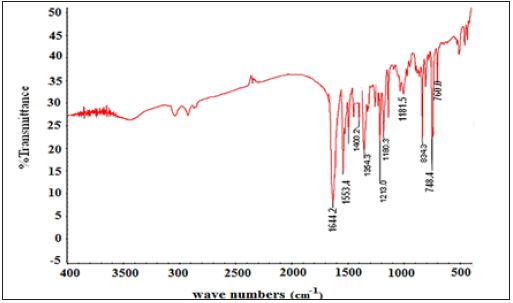
Infrared Spectroscopic Studies of bis (2-methoxy-1- napthaldehyde) hydrazine hydrate (BMNHH) and its derived Schiff based resin polymethylene, bis (2-methoxy-1-napthaldehyde) hydrazine hydrate (PMBMNHH). Fourier Transform Infrared (FTIR) plot of Schiff base BMNHH and resin PMBMNHH are showed in (Figure 4 & 5) correspondingly. The spectra of Schiff base clearly determine >C=N azomethine peak at about 1644cm-1, medium intensity peak. The FT-IR spectra of Schiff based resin showed strong band confirmed at 1650cm-1.The 6cm Shift of band in resin toward higher frequency higher wave number and abundant intensities absorption bands confirm the formation of resin PMBMNHH.
Figure 4: FT-IR spectra of schiff base BMNHH.
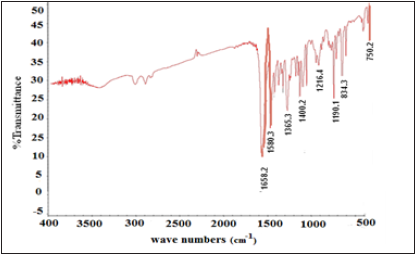
Figure 5: FT-IR spectra of schiff based resin PMBMNHH.
Nuclear magnet resonance 1H-NMR spectroscopy 1H-NMR spectroscopy of schiff base (BHNHH)
The 1H-NMR was recorded in DMSO on a 300MHz instrument showed in (Figures 6 & 7). A sharp singlet for OH displayed at δH 12.83 the maximum downfield signal of the spectrum. A new sharp singlet appearance like at δH 9.98 representing the presence of HC=N. H-8 and H-8′ appeared as doublet at δH 8.65 having coupling constant J8′,7′/8,7=8.4Hz, while H-4 and H-4′, appeared at δH 8.04 as doublet having coupling constant J 4′,3′/4,3=8.8Hz. Additional doublet for H-5′, H-5, appeared at δH 7.92, having coupling constant J5′,6′/5,6=8.0Hz. H-7′ and H-7resonated at δH 7.61 as triplet with coupling constant J7′,6′/7′,8′/7,6/7,8=7.8Hz. Another triplet of H-6′, H-6 resonated at δH 7.43, having coupling constant J6′,5′/6′,7′/6,5/6,7=7.4Hz. while H-3′ and H-3 appeared at δH 7.28 as doublet having coupling constant J3′,4′/3,4=8.8Hz.
Figure 6: 1HNMR spectrum of schiff base BHNHH.
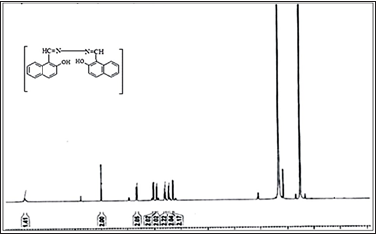
Figure 7: Represents the characteristic 1H-NMR signals of BHNHH.
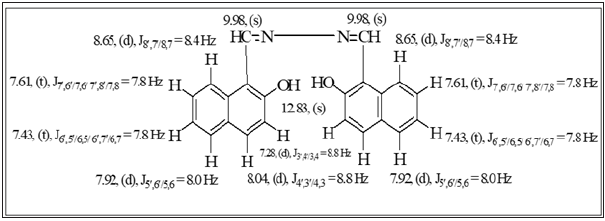
Electron impact ionization mass spectrometry EI-MS spectral studies of schiff base (BHNHH)
The spectrum of Schiff base BHNHH is showed in (Figure 8). The M+ at m/z 340 for molecular formula C22H16N2O2. The fragment appeared at m/z 170 presented that the molecule is cleaved into two equal halves with the formation of two 1-(iminomethyl) naphthalen-2-ol fragments, though, alternative fragment displayed at m/z 323 which exhibited the loss of an OH radical ion from the parent molecule, this fragmented upon further loss of 1-(iminomethyl) naphthalen-2-ol that appeared at m/z 154.
Figure 8: EI-MS spectrum of schiff base (BHNHH).
Thermogravimetric Analysis (TGA) studies of schiff base BMNHH and schiff based resin PMBMNHH
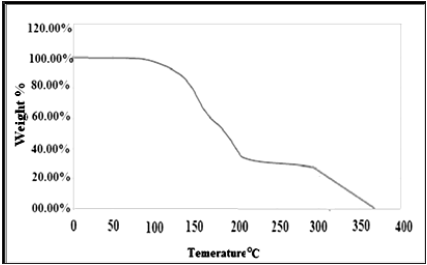
The thermal analysis of Schiff based BMNHH is shown in the (Figure 9), initial weight loss of 2% was observed between 50-100 ᵒC was due to loss of water in the sample. The material suffered rapid weight loss between 220 and 34 oC. The loss rate is 40% which is maximum rate of weight loss (Tmax).The thermal analysis of Schiff based resin PMBMNHH is displayed in the (Figure 10), initial weight loss of 2% was experiential 50-100 °C was due to loss of water in the sample. The material suffered rapid weight loss between 200 and 400 °C. The loss rate is 60% which is highest rate of weight loss (Tmax).
Figure 9: TGA spectra of schiff base PMBMNHH.
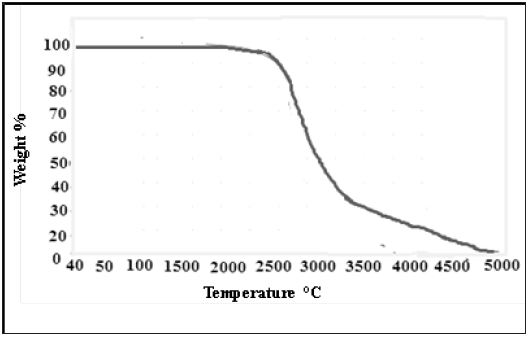
Figure 10: TGA spectra of schiff based resin PMBMNHH.
EDS analysis of schiff base BMNHH and schiff based resin PMBMNHH
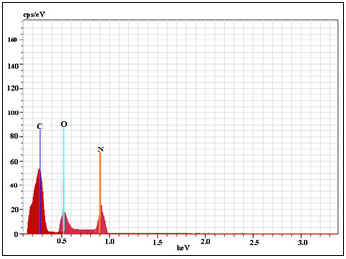
EDS spectrum and elemental composition demonstrates the schiff base BMNHH and schiff based resin PMBMNHH showed in (Figure 11 & 12) displayed that identified the material is mainly composed of carbon and oxygen as a esential material along with N as a constitute in the sample. EDS analysis shows a composition BMNHH of 72.60% C, 21.433% O, and 30.51% N (wt%) and Schiff based resin PMBMNHH shows a composition 75.18.% C, 23.54% O, and 34.75% N (wt. %) as evidence of successful inter-diffusion and the formation of compound.
Figure 11: EDS spectrum of schiff base BMNHH.
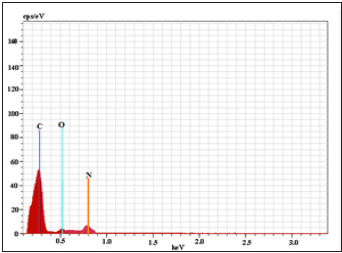
Figure 12: EDS spectrum of schiff based resin PMBMNHH.
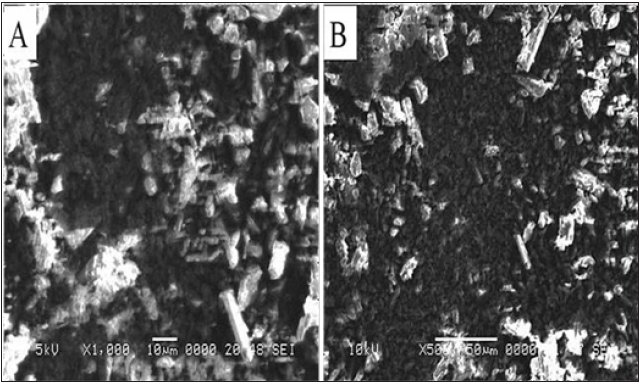
SEM analysis of schiff base (BMNHH) and schiff based resin (PMBMNHH)
The Schiff base BHNHH and resin PMBHNHH were subjected to SEM analysis. The topographic effects of BHNHH particles indicated obvious variation from sharp crystalline, longitudinal, irregular surface whereas the resin presented sub merged particles. It confirmed the formation of resin. In (Figure 13) represents the images of Schiff base BHNHH, at magnification×1000 indicating the irregular surface, spherical, longitudinal particles, having sharp crystal like structure. The diameter of Schiff base particles, in the range of 10 micron. In (Figure 14) showed the SEM images of resin PMBHNHH at magnification×500 indicated the strong binding and fused particles as compared to Schiff base.
Figure 13: SEM images of schiff base BHNHH.
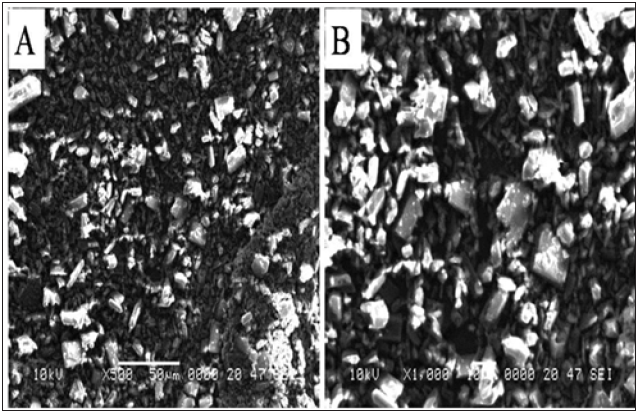
Figure 14: SEM images of resin PMBHNMXD.
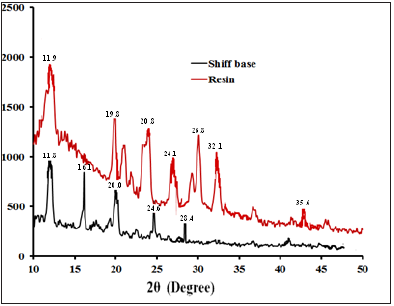
XRD analysis of Schiff Base BMNHH and Schiff Based Resin PMBMNHH
The diffraction pattern of Schiff base and Schiff based resin is indicated by black and red lined coolers respectively showed in (Figure 15). The X-ray diffraction pattern of Schiff Base BMNHH and Schiff Based Resin PMBMNHH are planes with peaks at 2θ, the peak 2θ=11.09 and 11.8 are associated with the (110), (111) planes, (Figure 11) it showed the crystallographic evaluation of Schiff base, and respective Schiff based resin. The black line in diffractrogram represents Schiff base BMNHH about 2θ angle around 11.8, 16.1, 20.0, 24.6 and 28.4 and. while red one represents is resin PMBMNHH about 2θ angle around, 11.9,19.8, 20.8, 24.1, 26.8, 32.1 and 35.6. The red line in diffractrogram represents the Schiff based resin, showed very sharp and high intense peaks compared to the Schiff base BMNHH. The Schiff based resin PMBMNHH showed 2θ angle around 11.9, 19.8, 20.8, 24.1, 26.8, 32.1 and 35.6, these peaks showed sharp spikes representing that the sample are crystal-like in nature, and are associated with the (110), (020), (101),(200),(210) and (221) planes, respectively. These reflection planes were approved and found in agreement with JCPDS card no: 7787-35-1 (The Powder Diffraction File (PDF) for orthorhombic structure). A very interesting result of Schiff based resin as compared to Schiff base was observed, displaying extra 232.1 and 35.6 peaks at 2θ. These additional and very sharp peaks were assigned to the (002) and (221) planes.
Figure 15: XRD analysis of schiff base BMNHH and schiff based resin PMBMNHH.
Biological studies
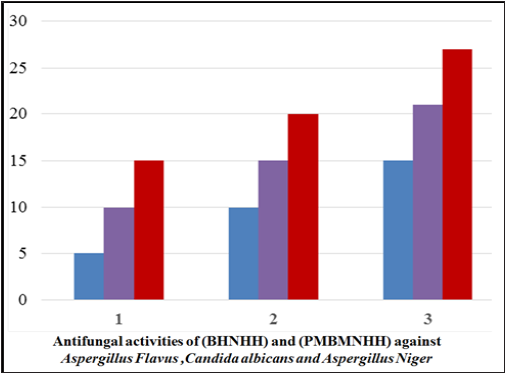
Antifungal activity of schiff base BHNHH and schiff based resin PMBHNH: The progress of antibiotics is great successes of recent science and technology for the control of fungal infections. The antifungal activities of Schiff bases and Schiff based resins were screened against the strains by using agar well diffusion method on Potato Dextrose Agar (PDA), which is a commonly used medium for growing fungus by a composition, water and potatoes. (Silica washed) used the fungal strains were grown on’’ PDA and remained kept for incubation at the temperature of 24±˚C for five days. The fungal slants were added with two to three milliliter’s of distilled water and after incubation at the said temperature, the cultures were filtered through ordinary whatman filter paper. Miconazole an antifungal was used as a standard drug. 10mg/ml of the test samples of (BHNHH) and (PMBHNHH) were used for recording the activity. The activity was observed through measuring colony diameter in millimeters of all the zones formed. The Schiff base and Schiff base resin showed inhibitory effects against cultures. The found results conveyed that the resin indicated enhanced activity as compared to its Schiff base against Aspergillus Flavus (Gram-negative), (5, 10, 15) Candida albicans (Gram-negative) (10, 20, 25) and Aspergillus Niger (Gram-negative) (15, 21, 27). The Schiff base showed zone of inhibition, whereas the resin exhibited zone of inhibition. The compound displayed sensitivity against all antifungal strains (Table 1) (Figure 16).
Table 1: Antifungal activity of schiff base (BHNHH) and schiff based resin (PMBHNHH).
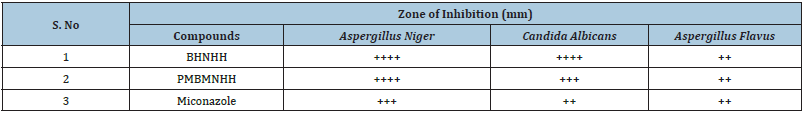
Source: Standard drug –ve control antifungal activity - = inactive, + = weakly active, ++ = moderately active, +++ = highly active ++++ = most active.
Figure 16: Bar graph representation of antifungal activities (BHNHH) and (PMBHNHH).
Conclusion
A novel Schiff base and Schiff based resin were prepared and successfully identified through CHN, FT-IR, EDS XRD and SEM analysis. The elemental analysis of synthetic compounds, were in agreement with the calculated values. The melting point enhancement was observed after recrystallization which improved the purity of compounds. The FT-IR spectra of Schiff based resin PMBMNHH showed strong absorption band at 1650cm-1 of azomethine >HC=N high intensities vibration than the Schiff base. This is the confirmation that structural arrangement of the synthesized resin. SEM analysis of Schiff based resin PMBMNHH indicated sharp crystalline, longitudinal, irregular surface. The X-ray diffraction configuration and crystallographic evaluation of Schiff base and its resin presented orthorhombic structure in each particle. The synthesized compounds successfully applied for antifungal activities. The activity was observed by measuring colony diameter in millimeters of all the zones formed. The Schiff bases and Schiff base resins showed inhibitory effects against cultures. The found results conveyed that the Schiff base and resin indicated enhanced activity against Aspergillus Flavus (Gramnegative), Candida Albicans (Gram-negative) and Aspergillus Niger (Gram-negative).The tested compounds show a good sensitivity against all antifungal strains.
References
- Joginder K, Amit R, Vinit R (2017) A comprehensive review on the pharmacological activity of schiff base containing derivatives. Organic & Medicinal Chem 1(3): 1-15.
- Sivasan k, Arish D, Johnson J (2016) Synthesis, characterization and biological studies on some metal complexes with Schiff base ligand containing pyrazolone moiety. Journal of Saudi Chemical Society 20: S591-S598.
- Ismet K, Ali B (2007) Synthesis, characterization, and thermal stability of azomethine oligomer and its metal complexes. Journal of Applied Polymer Science 105(3): 1356-1356.
- Xin Y, Sun W (2010) Synthesis and metal ion adsorption studies of chelating resins derived from macroporous glycidyl methacrylate-divinylbenzene copolymer beads anchored schiff bases. Journal of Applied Polymer Science 117(2): 953-959.
- Islam MN, Shahriar SMS, Jesmin M, Ali MM, Khanam JA, et al. (2013) Antibacterial activities of some transition metal schiff base complexes. International Letter of Chemistry Physics and Astronomy 10: 12-20.
- Sathis KD, Ibrahim AKS (2015) Synthesis, characterization, antibacterial activity and DNA cleavage studies of Schiff base Co (II) transition metal complexes. Der Pharma Chemica 7(11): 79-86.
- Kumar A, Fernandes J, Kumar P (2014) Synthesis, antimicrobial and anti-inflammatory studies of some novel schiff base derivatives. Int J Drug Dev Res 6:
- Sahu R, Thakur D, Kashyap P (2012) Schiff base: An overview of its medicinal chemistry potential for new drug molecules. International Journal of Pharmaceutical Science and Nanotechnology 5(3): 1757-1764.
- Monier M, Wei A, Sarhan A (2010) Adsorption of Cu(II), Hg(II), and Ni(II) ions by modified natural wool chelating fibers. Journal of Hazardous Materials 177(1-3): 348-355.
- Nura G,Hapipah MA, Hamid K, Mahmood AA, Hamid AH, et al. (2012) Antibacterial evaluation of some schiff bases derived from 2-acetylpyridine and their metal complexes. Molecules 17(5): 5952-5971.
- Syahrul I, Muhammad T, Nor H, Khalid M, Naz F, et al. (2014) Synthesis of novel bisindolylmethane schiff bases and their antibacterial activity. Molecules 19(8): 11722-11740.
- Roya R, Sara J (2015) Synthesis, characterization, and antibacterial activities of some metal complexes of heptadentate schiff base ligand derived from acetylacetone. Russian Journal of Applied Chemistry 88(6): 921-925.
- Kumar D, Sheriff AK (2015) Synthesis, characterization, antibacterial activity and DNA cleavage studies of Schiff base Co(II) transition metal complexes. Der Pherma Chemica 7(11): 79-86.
- Shalin K, Durga N, Saxena P (2009) Applications of metal complexes of schiff bases-A review. Journal of Scientific & Industrial Research 68: 181-187.
- Kumar A, Fernandes J, Kumar P (2014) Synthesis, antimicrobial and anti-inflammatory studies of some novel schiff base derivatives. Int J Drug Dev & Res 6(2): 165-171.
- Monier M, Ayad D, Wei Y, Sarhan A (2010) Adsorption of Cu(II), Co(II), and Ni(II) ions by modified magnetic chitosan chelating resin. Journal of Hazardous Material 177(1-3): 962-970.
- Suha k, Al Mosawi, Zeki A, Al Shamkhani (2016) New heterocyclic schiff base and azetidinone as antibacterial agents. International Journal of Environmental Chemistry and Ecotoxicology Research 1(3): 33-37.
- Naqvi A, Shahnawaaz M, Daya A, Seth S, Sharma N, et al. (2009) Synthesis of schiff bases via environmentally benign and energy-efficient greener methodologies. E-Journal of Chemistry 6(1): 75-78.
- Moina A, Mughal A, Zuhra M, Muhammad YK, Sumera Q (2013) Synthesis, characterization, viscosity and thermo analytical studies of schiff base polymers derived from 6,6-Methylene bis(1-napthaldehyde). Asian Journal Chemistry 25(17): 9931-9935.
- Mughal A, Mughal MA, Zuhra M, Muhammad Y, Nusrat NM (2013) Antimicrobial, thermoanalytical and viscometric studies of metal based schiff base polymer. J Chem Soc Pak 35(6): 1-8.
- Mughal A, Zuhra M, Muhammad Y, Akhtar M (2013) Synthesis and characterization of antimicrobial metal based polymeric ligand derived from 4,4'-Methylene-bis(furfuraldehyde). Asian Journal of Chemistry 25(11): 6327-6333.
© 2021 © Nasira Liaquat Rajput. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






