- Submissions

Full Text
Significances of Bioengineering & Biosciences
Influence of Conditions of GC- Flame Ionization Detector Analysis on Parameters of Peaks in Highly Concentrated Compounds
Ludmila Yarmolinsky1, Leonid Yarmolinsky2, Khalfin B1,3, Budovsky A4 and Ben-Shabat S3*
1Eastern R&D Center, Israel
2Arnie Miller Laboratories, Israel
3Faculty of Health Sciences, Ben-Gurion University of the Negev, Israel
4Research & Development Authority, Barzilai University Medical Center, Israel
*Corresponding author: Shimon Ben- Shabat, Faculty of Health Sciences, Ben- Gurion University of the Negev, Beer- Sheva, Israel
Submission: August 02, 2021 Published: August 31, 2021

ISSN 2637-8078Volume5 Issue2
Abstract
The aim of this research was to investigate an influence of flow velocity on the main parameters (retention time, tailing factor, relative area and number of theoretical plates) of peaks belonging to volatile compounds in the GC- Flame ionization detector analysis. Our results showed that as the flow increases, the retention time declines significantly (p<0.01). The other above-mentioned parameters of peaks also change depending on the characteristics of the solvents. The obtained results provide a platform for increasing accuracy of impurity tests for many volatile compounds and development of novel methods of analysis.
Keywords: GC-FID; Flow velocity; Tailing factor; Theoretical plate
Abbreviations: FID: Flame Ionization Detector
Introduction
GC-FID analysis is actively used for determination of purity of volatile compounds in environmental, clinical, pharmaceutical, biochemical, forensic, food science and petrochemical laboratories. The present study allows to optimize a set of conditions [1] for the development of new analytical methods for assessment of concentrations in chromatography. A direct injection of analyzed substances is one of the most widespread and the simplest methods applied [2,3]. The purity of compound is determined in accordance with the relative area of peak of the dominant compound in the mixture. In spite of the fact that various may give a certain inaccuracy, this method is simple in its realization and calculations, and is sufficiently widely used. One of the keys for the successful GC application is the geometrical clarity of the eluted peak boundaries [4]. The tailing factor is defined by the United States Pharmacopeia as the distance from the front edge of the peak to the back edge, divided by the distance from the front edge to the centerline, with all distances measured at 5% of the maximum peak height. In addition, the tailing factor must be close enough to 1 because it is an important prerequisite of correct functioning of majority kinds of software. When an injection of highly concentrated compound is performed, often the ideal form of the peak is not achieved. Dependence of number of theoretical plates (N) on the physical properties of the analyzed compounds was investigated many years ago, in the 60th years of previous century [5,6].
Despite the progress in the development of the chromatographic dynamic models, the studies on the conditions of the GC-FID analysis with regard to the parameters of eluted peaks are rare. Yet, the traditional research has tended to focus on the actual outcomes of the column estimation, rather than on the on the main parameters of peaks. For example, a general rate model permitted reasonably accurate predictions for plug flow through open columns, but it was inaccurate for pressure driven flow cases [7]; the volume averaging model was accurate for some columns [8]. To the best of our knowledge, the question of how conditions of analysis influence the number of theoretical plates was not investigated, as the number of theoretical plates was considered mainly for control of the efficiency of a column [9]. Influence of evolution factor on chromatography was also discussed [10]. Theoretically, a whole complex of physical properties of compounds has an impact on such parameters including the tailing factor and number of theoretical plates. These properties include boiling temperature, enthalpy and evaporation, polarity, dipole moment, viscosity, and specific interactions of the compound with the gas phase [1,2].
A main physical principal of GC chromatography is dynamic equilibrium of sorption/de sorption in the gas flow, the flow velocity is important ruling factor. Connection between retention time and boiling point of compounds is more or less clear but in what way parameters of the peak of the compound are related with properties of this compound is less understood. To the best of our knowledge, an influence of flow velocity on the main parameters of peaks was never considered in such way. The purpose of this study is to investigate the influence of flow velocity on the main parameters of peaks of volatile compounds (solvents) in GC-FID analysis.
Materials and Methods
Compounds
The analyzed compounds (1,4-Dioxane, N,N Dimethylformamide, N,N Dimethylacetamide, Cyclohexanone, Methyl-Ethyl Ketone, N-Propanol, Dichloromethane, Ethyl acetate) were purchased from Merck, Kenilworth, N.J., U.S.A.
GC-FID analysis
Varian GC-800 analytical system was applied with FID detector. The split/splitless liner was int d2mm. A carrier gas was helium UHP. A column (Cyanomethyl- Phenylsilane), 75m×0.53mmi.d was used.
Statistical analysis
Independent experiments were repeated three times. All data were analyzed using Statistica for Windows software (Stat Soft, Inc., Tulsa, OK), and p<0.05 was chosen as the minimal acceptable level of significance. Simple regression models were subsequently used to eliminate non-significant effects. Values are presented as means ±SD.
Result and Discussion
Physical properties of the of the analyzed compounds were collected (Table 1). The above-mentioned compounds were selected for GC- Flame Ionization Detector Analysis because of the following reasons: (a) these compounds represent an interesting model for analysis because they have different boiling points and relative polarities (Table 1); (b) these compounds are the most frequently used for impurity test. The novelty of the proposed study lies at estimating the influence of flow velocity on the main parameters of peaks of volatile compounds. (Table 2) demonstrates that once the flow increases, the retention time decreases significantly (p<0.01). The approximately linear relation is observed for N-Propanol, Methyl-Ethyl Ketone and Ethyl acetate, but it is highly different from 1 for 1,4-Dioxane, N, N Dimethylformamide, N, N Dimethylacetamide, Cyclohexanone and Dichloromethane. Abnormally high value of the retention time is observed for Dichloromethane at boiling temperature that may be explained by the interaction of its molecules with column phase. The movement of the compounds in the column is determined by three main factors. The first factor is an interaction of the compounds with the active phase of the column mainly because of sorption and as a result of Van der Waals interaction. The second factor is the desorption by means of the column temperature. A balance between the sorption and the desorption depends directly on the column temperature, and indirectly on flow velocity by means of forming the concentration gradient between the sorption and the desorption phases. The third factor is the front migration of the material at the maximal concentration which is proportional to the flow velocity. The retention time is described as (upon condition of constant temperature)
Table 1: Boiling point, relative polarity and topological tolar surface area of the of the analyzed compounds.
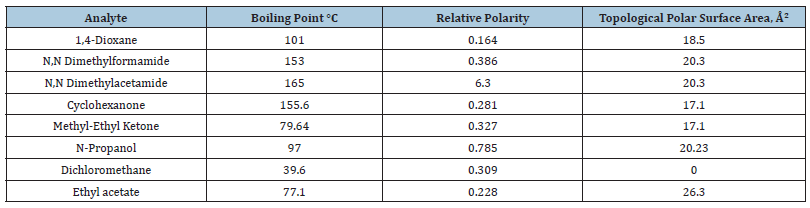
Table 2: Flow, retention time and area of the analyzed compounds.
RT∼1/Flow+⟨o⟩
where RT- Retention Time, o- Irrelevant Factor or as
RT∼1/Flow+⟨O⟩
Where O-relevant factor.
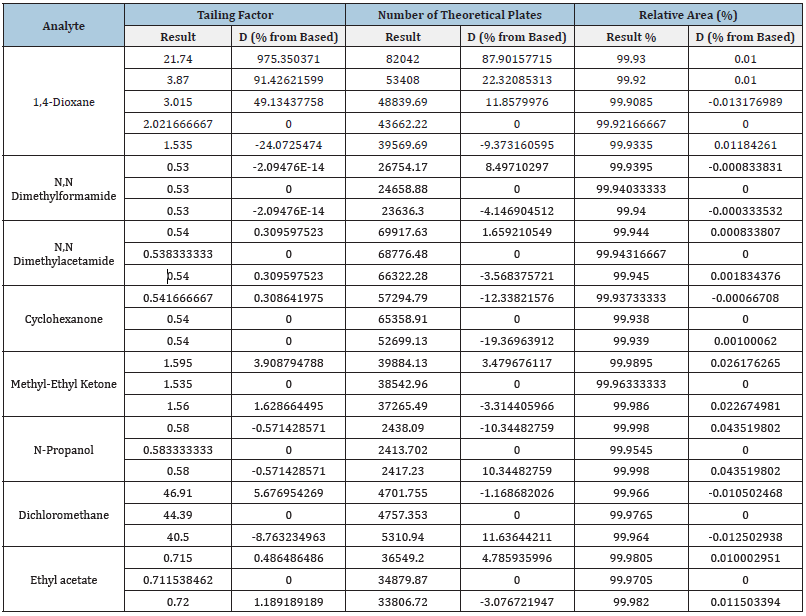
If it is dRT/dFlow < 0, Then dRT/dFlow → const, but if it is dRT/dFlow ≠ const the strong interaction of the compounds with the column material is observed. In this case the compound cannot be retained in the gas phase. The tailing factor depends on the flow in different ways. For example, there is a significant decrease in the length of the tailing factor (p<0.01) in cases of 1,4-dioxane and dichloromethane which was not observed for other tested compounds (Table 3). Of note, 1,4-dioxane and dichloromethane are molecules that have two highly electronegative centers, and they are symmetrical. There is no significant dependence between the flow rate and the relative area of the main peak (Table 3).
The number of theoretical plates depends on the flow that is connected with polarity and properties of the compounds. For N-Propanol and Methyl-Ethyl Ketone these dependencies are the same, while in case of N, N Dimethylformamide and N, N Dimethylacetamide (the compounds with similar structures) the number of theoretical plates depends on the flow qualitatively, but not quantitatively (Table 3).
Table 3:Tailing factor, number of theoretical plates and relative area of the analyzed compounds.
Concluding Remarks
Our results show that as a result of flow increase, the retention time declines. The remaining parameters of peaks vary according to the properties of the solvents. The obtained results may allow increasing the accuracy of impurity test for many volatile compounds, and may facilitate development of novel methods of analysis for impurity test by understanding of ruling parameters. The further investigations are important in order to elucidate the influence of the column phase and the temperature gradient on the main parameters of peaks of volatile compounds in the GC-FID analysis.
References
- Pollock GS, Eldridge RB (2000) Neural network modeling of structured packing height equivalent to a theoretical plate. Ind Eng Chem Res 39: 1520-1525.
- Babuška I, Li L (1992) The problem of plate modeling: Theoretical and computational results. Computer Methods in Applied Mechanics and Engineering 100(2): 249-273.
- Velayudhan A, Ladisch MR (1993) In: Tsao GT (Ed.), Springer Berlin Heidelberg, Berlin, Germany, pp. 123-145.
- Shou M, Qiu H (2020) Development of a rapid GC-FID method to simultaneously determine triethylamine, diisopropylamine, and 1,1,3,3-tetramethylguanidine residues in an active pharmaceutical ingredient. Journal of Pharmaceutical Analysis 11(2): 251-256.
- Wicke E (1967) J. C. Giddings: Dynamics of Chromatography. Part. I: Principles and Theory. Marcel Dekker, New York, 1965. XII und 323 Seiten. 39 Abb. Preis:$ 11.50. Reports of the Bunsen Society for Physical Chemistry 71(2): 236-236.
- Kucera E (1965) Contribution to the theory of chromatography: Linear non-equilibrium elution chromatography. J Chromatogr 19(2): 237-248.
- Lieres E, Andersson J (2010) A fast and accurate solver for the general rate model of column liquid chromatography. Computers & Chemical Engineering 34(8): 1180-1191.
- Yan X, Wang Q (2013) Comparative analysis of chromatography dynamic models in predicting the plate height contributed by interphase mass transfer. Chemical Engineering Science 104: 760-766.
- Stauffer E, Dolan JA, Newman R (2008) Fire Debris Analysis. (1st), Academic Press, USA, pp. 1-672.
- Kaiser RE (1977) The correct measurement and interpretation of evaluation factors in chromatography: The real plate number, the separation number, the dosage quality. Part 1: Gas chromatography. Chromatographia 10(6): 323-338.
© 2021 © Ben-Shabat S. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






