- Submissions

Full Text
Significances of Bioengineering & Biosciences
Impact on Fundamental Physiological Functional Biomarkers after Administration of Biofield Treated Formulation in Unpredictable Chronic Stress (UCS)-Induced Rats
Mahendra KT1, Alice Branton1, Dahryn Trivedi1 and Snehasis Jana2*
1Trivedi Global Inc, USA
2Trivedi Science Research Laboratory Pvt. Ltd., India
*Corresponding author: Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd., India
Submission: July 13, 2021 Published: August 18, 2021

ISSN 2637-8078Volume5 Issue2
Abstract
The aim of the present study was to evaluate the fundamental physiological functional biomarkers analysis of biofield treated/blessed novel test formulation in Unpredictable Chronic Stress (UCS) male Sprague Dawley (SD) rats. The constituents of the test formulation were divided into two parts; one section was defined as the untreated test formulation, while the other part of each components and three group of animals received biofield energy healing/blessing treatment by Mr. Mahendra Kumar Trivedi, a famous biofield energy healer. Total Leucocyte Count (TLC) count was significantly (p≤0.001) improved by 75.62%, 64.20%, 44.03%, 82.53%, and 50.83% in the biofield energy treated test formulation to the untreated rats (G5), biofield energy treatment per se to the rats (G6) groups, 15 days pre-treatment of biofield energy treated test formulation (G7), 15 days pre-treatment of biofield energy treated test formulation to the biofield energy treatment per se to rats (G8), and untreated test formulation to the biofield energy treated rats (G9) respectively, as compared with the G2 group. Besides, lymphocytes count were significantly increased by 52.50% (p≤0.001), 47.02% (p≤0.001), 32.21%, 83.86% (p≤0.001), and 40.15% (p≤0.001) in the G5, G6, G7, G8, and G9 groups, correspondingly with reference to G2 group. In addition, monocyte counts were also significantly increased by 82.52% (p≤0.05), 65.06% (p≤0.05), 38.68%, and 17.03% in the G5, G6, G8, and G9 groups, respectively as compared with the G2 group. Biochemical analysis showed that glucose level was maintained in all the experimental test groups, while UCS significantly increased the level of glucose. Biochemical analysis showed that HDL level was significantly (p≤0.001) improved by 37.62%, 46.64%, and 36.27% in the G5, G7, and G9 groups, respectively as compared with the G2. However, total cholesterol was decreased by 14.86%, 30.32%, 44.29%, and 32.66% in the G5, G7, G8, and G9 groups, correspondingly with reference to G2. However, VLDL level was also significantly reduced by 14.91%, 30.70%, 44.30% (p≤0.01), and 32.89% in the G5, G7, G8, and G9 groups, respectively as compared with the G2. Hepatic and cardiac biomarkers analysis showed that ALP level was decreased by 27.97% and 13.37% in the G5 and G9 groups, respectively as compared with the G2. The level of SGOT was significantly (p≤0.01) decreased by 21.45%, 19.19%, and 19.81% in the G5, G7, and G9 groups, respectively as compared with the G2. On the other hand, SGPT level was also altered by 11.32% and 11.72% in the G5 and G6 groups, respectively with reference to G2 group. CK-MB level was significantly reduced by 16.32% and 37.01% (p≤0.01) in the G8 and G9 groups, respectively with reference to G2. Animal weight parameters like body weight, organ to body weight ratio, and feed intake data showed that the changes were non-significant and no toxic effect was observed after the experimental period. Overall, the data suggested a significant improvement of immune-related hematology parameters, CK-MB, SGOT, and lipid profile after biofield treatment and slowdown the disease progression rate and related symptoms in the preventive maintenance groups. Therefore, the data could be benefitted to build-up good body immune responses, enhance resistance towards diseases, and reduce allergies and lethargic conditions.
Keywords: Biofield treatment; Haematology; Biochemical analysis; The Trivedi Effect®; Unpredictable chronic stress
Abbreviations: NCCAM: National Center for Complementary and Alternative Medicine; NCCIH: National Centre of Complementary and Integrative Health; CBD: Cannabidiol; CPCSEA: Control and Supervision of Experiments on Animals; TLC: Total Leukocyte Count; DLC: Differential Leukocyte Counts; LDL: Low Density Lipoprotein; HDL: High Density Lipoprotein; VLDL: Very Low Density Lipoprotein; TC: Total Cholesterol; TG: Triglycerides; UCS: Unpredictable Chronic Stress; SD: Sprague Dawley; CBD: Cannabidiol Isolate; CPCSEA: Control and Supervision of Experiments on Animals; ALP: Alkaline Phosphatase; SGOT: Serum Glutamic Oxaloacetic Transaminase; CK-MB: Creatine Kinase Myocardial Band; SEM: Standard Error of Mean
Introduction
The state of stress could be considered as occurring when there are various internal and external factors present that negatively affect the homeostatic equilibrium of the body of organisms from its molecular level to the whole-body level. Stress might impose a severe effect on the organism’s welfare status by inducing the energyconsuming mechanisms of the body to combat the subsequent ill effects, which further may immune-compromise the individual and thereby making them vulnerable to pathogens [1]. Besides, there are various indicators that may serve the purpose as potent markers of different biological processes, known as biomarkers, which denote any pathogenic or pharmacological responses of the body [2-5]. In this regard, there are various normal physiological biomarkers, which denote the healthy status of an individual, if present within the normal range. However, the presence of stress marker indicates the physiological uncomforting of the individual that might results due to operation of different energy consuming mechanisms within the body in the process of maintaining the homeostasis and thereby involves numerous biomarkers [6,7]. Moreover, the changes in the body due to stress involves various physiological and endocrine alterations, along with disturbances in the corresponding functional, biochemical, and metabolic systems and thereby, the alterations in the metabolic biomarkers such as the enzymes, metabolites, and hormones, etc., also result [8,9]. Furthermore, such alterations consequently affect the other vitals of the body such as cardiovascular, renal, and CNS system, which are early responding, followed by the hepato-biliary and pancreatic systems, which are considered as late responding. There are various biomarkers concerning the cardio-vascular-metabolic function of the body such as, those of vascular function (e.g., FMD, BP, AIX), vascular cytokines, vasculature (e.g., HDL, cholesterol, LDL, sICAM, SAA, sVCAM), magnesium, homocysteine, and cardiac (e.g., troponins, myeloperoxidase (MPO), C-reactive protein, natriuretic peptides), etc. [10,11]. Similarly, there are biomarkers that denote the imbalance in the corresponding metabolic parameters, liver enzymes, kidneys biomarkers, secondary messengers in brain, and specific other biomarkers, etc. have been enumerated [12-14]. During chronic stress, the organism is subjected to a prolonged stressor, which creates irregular energy homeostasis that might continue for a long duration of time and therefore, considered detrimental. Such irregularities may further cause the metabolic disturbances such as cardiovascular disease, obesity and type-II diabetes mellitus, etc. On the basis of this concept, the scientists have started extensive studies using animal models, and also reported the neurobiology of stress-induced metabolic disorders. This study also involved various rodent stress models that further helps in determining the factors that might affect the metabolic outcome [15,16], thereby affecting their body weights, organ weights, and other haematological and biochemical profile. In this study the effect of Unpredictable Chronic Stress (UCS) on immune system of male Sprague Dawley rats was studied in presence of novel test formulation, which contains the combination of minerals such as, iron, selenium, zinc, copper, calcium, and magnesium; vitamins such as cyanocobalamin, pyridoxine HCl, ascorbic acid, alpha tocopherol, and cholecalciferol; along with β-carotene, Ginseng, and Cannabidiol Isolate (CBD).
Besides, the test formulation is treated with Biofield Energy Treatment by a renowned Biofield Energy Healer. The Biofield Energy Healing is a kind of Complementary and Alternative Medicine (CAM) therapy, which involve the healing process using the energy therapy. Moreover, the energy healing therapies are known for their beneficial effect against many diseases, therefore, accepted by the National Center for Complementary and Alternative Medicine (NCCAM) under CAM therapies [17,18]. The biofield energy healing therapy is based on the fact that a human has the ability to harness energy from the universe and can transmit it to any living organism(s) or non-living object(s) around the globe. There are various other CAM health care approach similar to the Biofield Energy Healing that are accepted by National Centre of Complementary and Integrative Health (NCCIH) such as therapeutic touch, yoga, Reiki, guided imagery, pranic healing, chiropractic/ osteopathic manipulation, hypnotherapy, mindfulness, meditation, Ayurvedic medicine, and traditional Chinese herbs and medicines in biological systems [19,20], etc. Moreover, there are various scientific literatures that reported the beneficial impact of the Trivedi Effect®- consciousness energy healing treatment in the field of materials science [21,22], agriculture science [23], antiaging [24], gut health [25], nutraceuticals [26], pharmaceuticals [27], overall human health and wellness. Thus, the present study was designed to scientifically evaluate the impact of the biofield energy treatment/blessing (the Trivedi Effect®) on the given novel test formulation and biofield energy treatment per se to the animals for its major haematological and biochemical biomarkers.
Material and Methods
Chemicals
Panax ginseng extract and Cannabidiol (CBD) isolate were obtained from Panacea Phyto extracts, India and Standard Hemp Company, USA, respectively. Magnesium (II) gluconate, β-carotene (retinol, provit A), pyridoxine hydrochloride (vitamin B6), zinc chloride, and calcitriol were purchased from TCI, Japan. Imipramine hydrochloride was purchased from Sigma, USA. Calcium chloride, copper chloride, cyanocobalamin (vitamin B12), iron (II) sulfate, cholecalciferol (vitamin D3), vitamin E (alpha-tocopherol), and sodium carboxymethyl cellulose (Na-CMC) were procured from Sigma-Aldrich, USA. Sodium selenate and ascorbic acid (vitamin C) and were obtained from Alfa Aesar, India.
Study design
The study was designed as such to fulfil the study protocol, animals (n=6) were assigned into nine (9) groups. G1: Normal control; G2: Disease control (UCS: Unpredictable Chronic Stress+0.5% CMC); G3: Reference item (UCS+Imipramine hydrochloride 30mg/kg); G4: (UCS+Untreated test formulation); G5: (UCS+Biofield Energy Treated test formulation); G6: (UCS+Biofield Energy Treatment per se to animals from day -15; G7: (UCS+Biofield Energy Treated test formulation from day-15); G8: (UCS+Biofield Energy Treatment per se+Biofield Energy Treated test formulation from day-15), and G9: (UCS+Biofield Energy Treatment per se animals+untreated test formulation).
Maintenance of animal
Randomly breed male Sprague Dawley (SD) rats with body weight ranges from 200 to 300gm were used in this study. The animals were purchased from M/s. Vivo Bio Tech, Hyderabad, India. Animals were randomly divided into nine groups based on their body weights consist of 6 animals of each group. They were kept individually in sterilized polypropylene cages with stainless steel top grill having provision for holding pellet feed and drinking water bottle fitted with stainless steel sipper tube. The animals were maintained as per standard protocol of the committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Govt. of India. The test facility is registered (registration no. 64/PO/br/s/99/CPCSEA) for animal experiments with the CPCSEA. The animals were procured using protocol approved by the Animal Ethics Committee (IAEC/41/505) and the husbandry conditions were maintained as per the recommendations of the CPCSEA.
Consciousness energy healing strategies
Each ingredient of the novel test formulation was divided into two parts, one part was not received any sort of treatment and were defined as the untreated or control sample. The second part of the test formulation was treated with the Trivedi Effect®- Energy of consciousness healing/blessing treatment (Biofield Energy Treatment) by a renowned biofield energy healer, Mr. Mahendra Kumar Trivedi under laboratory conditions for ~3 minutes. Besides, three group of animals also received biofield energy healing treatment (known as the Trivedi Effect®) by Mr. Mahendra Kumar Trivedi under similar laboratory conditions for ~3 minutes. The Biofield Energy Healer was located in the USA, however the test formulation were located in the research laboratory of Dabur Research Foundation, New Delhi, India. The energy transmission was done remotely to the samples or animals. After that, the biofield energy treated/blessed samples was kept in the similar sealed condition and used as per the study plan. In the same manner, the control test formulation group was subjected to “sham” healer for ~3 minutes energy treatment, under the same laboratory conditions. The “sham” healer not has any knowledge about the biofield energy treatment. The biofield energy treated/ blessed animals were also taken back to experimental room for further proceedings.
Experimental details
Animals were randomized after seven (7) days of acclimatization, and grouped according to their body weight (b.w.). The test formulation was prepared freshly before administration to the animals using an oral intubation needle that is attached to a graduated disposable syringe. The dose volume was given as 10mL/kg in the morning and evening as per b.w. The experimental groups were divided into G1 to G9. G1 and G2 animals were treated with 0.5% w/v CMC-Na in distilled water orally for 8 weeks. Group G3 animals was treated with reference item, imipramine hydrochloride orally at a dose of 30mg/kg, b.w. for 8 weeks. The freshly prepared suspensions of the biofield energy treated and untreated test formulation was administered orally to the G4 and G5 animals @1257.80mg/kg, b.w. (morning) and 2012.75mg/kg, mg/kg, b.w. (evening), respectively for 8 weeks. G6 group was not to be dosed with the test formulation. Additionally, G7, G8, and G9 groups were provided similar to the G4 and G5 dosing regimen, but from the day of biofield energy treatment (i.e., from day -15 to day 56). Body weight and clinical signs were taken daily throughout the experimental period. All the animals except G1 group received stress-induced procedures (sound stress, tilted cages and crowd stress, cold and warm water swim stress, feed and water deprivation, stress due to change in the light and dark cycle) were undergo seven different types of unpredictable stress procedures after scheduled dosing daily at specified interval to the end of the experiment for 8 weeks. After the initiation of stress, which vary every week interval i.e., shuffling of stress type. At terminal (8 week) experimental period, all the animals were individually subjected for blood collection using retro-orbital route to the experimental purpose such as hematology and biochemistry.
Assessment of hematology parameters in blood
The blood parameters like Total Leukocyte Count (TLC) and Differential Leukocyte Counts (DLC) were analyzed using Hematology analyzer (Abbott Model-CD-3700) in blood samples [28,29].
Assessment of lipid profile and glucose in serum
The test formulation was tested for important lipid biomarkers such as Total Cholesterol (TC), Triglycerides (TG), Low Density Lipoprotein (LDL), High Density Lipoprotein (HDL), Very Low Density Lipoprotein (VLDL), and glucose were analyzed using serum by Biochemistry Analyzer, Spectralab A-plus, Italy [30]. Assessment of hepatic and cardiac biomarkers in serum The test formulation was tested for important hepatic and cardiac biomarkers such as Alkaline Phosphatase (ALP; U/L), Serum Glutamic Oxaloacetic Transaminase (SGOT; U/L), Serum Glutamic Pyruvic Transaminase (SGPT; U/L), Creatine Kinase Myocardial Band (CK-MB; U/L) albumin (g/dL), globulin (g/dL), A/G ratio, total bilirubin (mg/dL), total protein (g/dL), and were analyzed using serum by Biochemistry Analyzer, Spectralab A-plus, Italy [31,32].
Determination of body weight, feed intake, and organ weight parameters
All the experimental animals were daily analyzed for their change in body weight, feed intake, which was calculated by weighing the daily feed supply and the left-over amount that evaluate the average daily feed intake. The average intake of feed was recorded in every three days interval throughout the experimental period. After terminal bleeding, the animals were sacrificed and the following organs such as liver, lungs, kidney, brain, heart, eyes, pancreas, spleen, thymus, adrenal gland, intestine, and reproductive organs, i.e., testis, prostate, epididymis and vas deferens were collected. These organs were trimmed off any adherent tissue and fat, as appropriate and weighed. The organ to body weight ratio percentage was identified by comparing the weight of each organ with the final body weight of individual rat. All the data were reported through the study treatment regimen. Relative organ weight was calculated as per Equation 1.
Clinical sign and symptoms
All the animals in different test groups were analyzed for various clinical signs and symptoms in accordance with in-house protocol. Abnormal behaviour in animals was recorded with the time of onset and disappearance.
Statistical assessment
The data were represented as mean±Standard Error of Mean (SEM) and subjected to statistical analysis using Sigma-Plot statistical software (Version 11.0). For multiple comparison oneway analysis of variance (ANOVA) followed by post-hoc analysis by Dunnett’s test and for between two groups comparison Student’s t-test was performed. The p≤0.05 (n=6) was considered as statistically significant.
Results and Discussion
Evaluation of haematological parameters
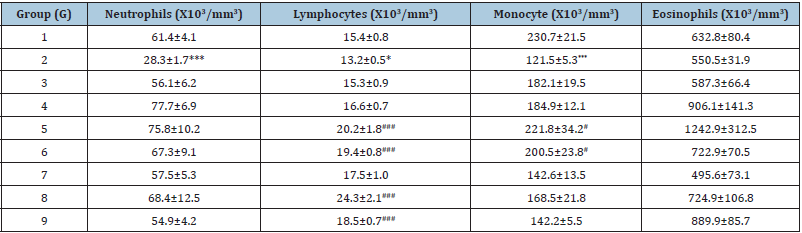
The experimental results showed an important haematology profile in different groups (G1 to G9), which are summarized in (Table 1). The study results suggest that biofield energy treated test formulation showed an improved animal hematology profile as compared with the disease control group. The results showed significantly decreased the levels of TLC, neutrophils, lymphocytes, and monocyte by 28.12% (p≤0.001), 53.97% (p≤0.001), 14.15% (p≤0.05), and 47.33% (p≤0.001), respectively, in the G2 group as compared with the normal control (G1) group. Hematology parameters such as TLC count was significantly (p≤0.001) increased by 75.62%, 64.20%, 44.03%, 82.53%, and 50.83% in the G5, G6, G7, G8, and G9 groups, respectively with reference to G2 group (Figure 1). Similarly, 52.50% (p≤0.001), 47.02% (p≤0.001), 32.21%, 83.86% (p≤0.001), and 40.15% (p≤0.001) significantly increased level of lymphocytes was reported in the G5, G6, G7, G8, and G9 groups, correspondingly with reference to G2 group. However, monocytes were also found to be significantly increased by 82.52% (p≤0.05), 65.06% (p≤0.05), 7.37%, 38.68%, and 17.03% in the G5, G6, G7, G8, and G9 groups, respectively with reference to G2 group (Table 1). It suggests that the due to chronic stress, significantly reduced the levels of TLC, neutrophils, lymphocytes, and monocyte, which were significantly managed after treatment with the Biofield Energy Healing Treatment has the capacity to improve the blood immunityrelated parameters. Thus, overall haematology parameters showed an improved major immune blood marker. T and B cells acts to eliminate the antigen, either by releasing the antibodies (B cells), cytotoxic granules or directly by signalling to other immune cells. Several growth factors are regulated in response to any infections, which was governed by the immune system [33]. Similarly, reduced level of neutrophils and monocytes can be correlated in presence of many chronic inflammatory diseases such as gout, rheumatoid arthritis, rheumatic fever, etc., which can be increased by any form of chronic stress factors. Overall, the biofield energy treated test formulation significantly improved the concentrations of TLC, lymphocytes, and monocytes in hematology profile assay, which suggest that the Trivedi Effect® has the capacity to improve the immunomodulatory potential of the novel proprietary test formulation.
Figure 1: Elevation of Total Leukocyte Count (TLC) after dosed of biofield blessed novel proprietary formulation and biofield blessing directly to the male Sprague Dawley rats. G: Group; G1: Normal control; G2: Disease control (UCS: Unpredictable Chronic Stress+0.5% CMC); G3: Reference item (UCS+Imipramine hydrochloride 30mg/kg); G4: (UCS+Untreated Test Formulation); G5: (UCS+biofield energy treated test formulation); G6: (UCS+biofield energy treatment per se to animals from day-15; G7: (UCS+biofield energy treated test formulation from day- 15); G8: (UCS+biofield energy treatment per se+biofield energy treated test formulation from day-15), and G9: (UCS+biofield energy treatment per se animals from day-15+untreated test formulation). Values are presented as mean±SEM (n=6). Source: ###p≤0.001 vs. G1 and ***p≤0.001 vs. G2.
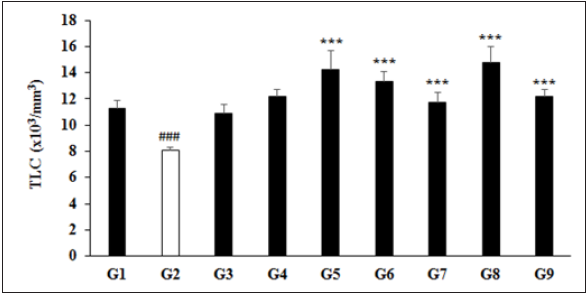
Table 1: Effect of the test formulation and per se blessing to the animals on the level of haematological parameters in male Sprague Dawley rats. Source: ***p≤0.001 vs. G1, *p≤0.05 vs. G1, ###p≤0.001 vs. G2 and #p≤0.05 vs. G2.
Measurement of glucose and lipid biomarkers
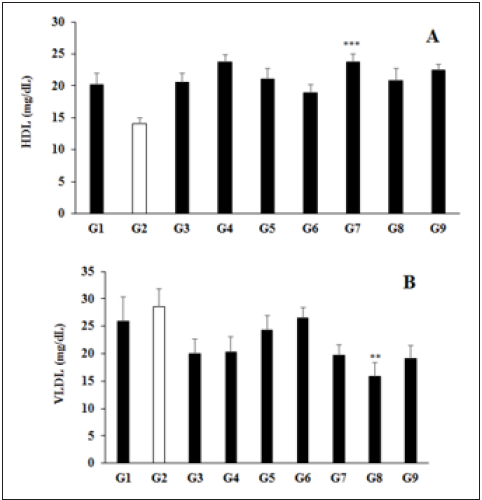
Lipid biomarker analysis was performed after treatment with the biofield energy treated and untreated test formulations are summarized in the (Table 2). The analyzed glucose and lipid biomarkers were tested such as Total Cholesterol (TC), Triglycerides (TG), High Density Lipoprotein (HDL), Low Density Lipoprotein (LDL), and Very Low Density Lipoprotein (VLDL). The experimental data showed that the concentration of glucose was increased by 28.55% in UCS group (G2) as compared with the G1, which was significant reduced and maintained in all the experimental test groups as compared with the disease control group (G2). However, the level of HDL was significantly (p≤0.001) increased by 37.62%, 46.64%, 34.46%, and 36.27% in the G5, G7, G8, and G9 groups, respectively as compared with the G2 group (Figure 2A). Similarly, TC was reduced by 14.86%, 7.17%, 30.32%, 44.29%, and 32.66% in the G5, G6, G7, G8, and G9 groups, correspondingly with reference to G2 group (Table 2). However, VLDL level was also significantly reduced by 14.91%, 7.02%, 30.70%, 44.30% (p≤0.01), and 32.89% in the G5, G6, G7, G8, and G9 groups, respectively as compared with the G2 group, while G8 group showed decreased level of VLDL by 21.60% as compared with the G4 (Figure 2B). Therefore, the biofield energy treated test formulation showed an altered lipid profile, which might be useful in immunomodulation. Biofield energy healing treatment has the significant capacity to improve the overall lipid profile that showed that suggests that biofield energy treated test formulation can be used to improve the lipid profile. The test formulation components such as vitamins, minerals, and important plant extracts have been reported with significant improved lipid profile, serum cholesterol, LDL, HDL, etc. [34-36]. Therefore, biofield energy healing treatment and biofield energy treated test formulation significantly regulates the liver lipid profile after oral administration of biofield energy treated/blessed test formulation, which might suggest its role in immunomodulation against many autoimmune and inflammatory disorders.
Table 2: Lipid profile analysis after treatment with Biofield Energy to the test formulation and animals per se in male Sprague Dawley rats. Source: TC: Total Cholesterol; LDL: Low Density Lipoprotein.
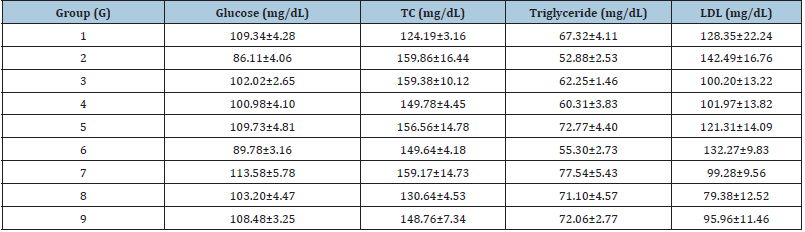
Figure 2: Elevation of High-Density Lipoprotein (HDL)-2A and lowering of Very Low-Density Lipoprotein (VLDL)- 2B after dosed of biofield treated/blessed novel proprietary formulation and biofield energy blessing directly to the male Sprague Dawley rats. Source: **p≤0.01 and ***p≤0.001 vs. G2.
Evaluation of hepatic and cardiac biomarkers
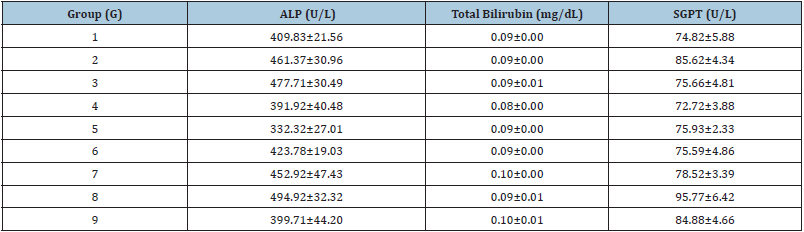
Hepatic and cardiac biomarkers data suggested significant improved level of biomarkers (Table 3) such as Alkaline Phosphatase (ALP) level was decreased in the experiment by 15.05%, 27.97%, 8.15%, 1.83%, and 13.37% in the G4, G5, G6, G7, and G9 groups respectively, as compared with the G2 group, while ALP was also decreased by 15.21% in the G5 group as compared with the G4 (Table 3). Similarly, SGOT level was significantly decreased by 27.15% (p≤0.01), 21.45% (p≤0.01), 13.22%, 19.19% (p≤0.01), 5.99%, and 19.81% (p≤0.01) in the G4, G5, G6, G7, G8, and G9 groups, correspondingly with reference to G2 group (Figure 3A). However, SGPT level was also maintained by 11.32%, 11.72%, and 8.30% in the G5, G6, and G7 groups respectively with reference to G2 (Table 3). CK-MB level was significantly reduced by 6.48%, 16.32%, and 37.01% (p≤0.01) in the G7, G8, and G9 groups, respectively as compared with the G2 group (Figure 3B). The globulin level was significantly (p≤0.001) increased by 12.89%, 16.72%, 15.68%, and 10.80% in the G6, G7, G8, and G9 groups, respectively with reference to G2 group. Further, total protein level was significantly (p≤0.01) increased by 6.24%, 6.24%, and 5.91% in the G6, G7, G8 groups, respectively with reference to G2 group (Figure 4). Hepatic enzymes were improved in the present study and they are the major biomarkers that suggest liver toxicity [37]. The test formulation components have been reported to have significant action on hepatic enzymes [38-40], thus the test formulation have showed an improved hepatic and cardiac biomarkers after treatment with the biofield energy treatment. Consequently, it can be concluded that the Trivedi Effect®-Biofield Energy Healing can be used to improve the immunity profile by improving hepatic and cardiac enzymes.
Figure 3: Downgrading of Serum Glutamic Oxaloacetic Transaminase (SGOT)-3A and reduction of creatine kinase myocardial band (VLDL)-3B after dosed of biofield treated/blessed novel proprietary formulation and biofield energy blessing directly to the male Sprague Dawley rats. Source: **p≤0.01 vs. G2.
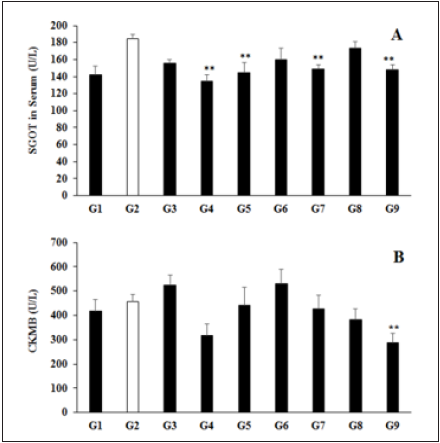
Figure 4: Comparative levels of Albumin (A), Globulin (G), total protein and their ratio (A/G) after dosed of biofield treated/blessed novel proprietary formulation and biofield energy blessing directly to the male Sprague Dawley rats. Source: **p≤0.01 vs. G2 and ***p≤0.001 vs. G2.
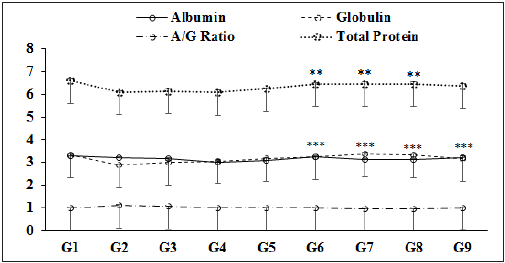
Table 3: Evaluation of hepatic biomarkers after treatment with the biofield energy to the test formulation and animals per se on male Sprague Dawley rats. Source: SGPT: Serum Glutamic Pyruvic Transaminase; ALP: Alkaline Phosphatase.
Estimation of animal weight parameters, feed intake, and relative organ weight
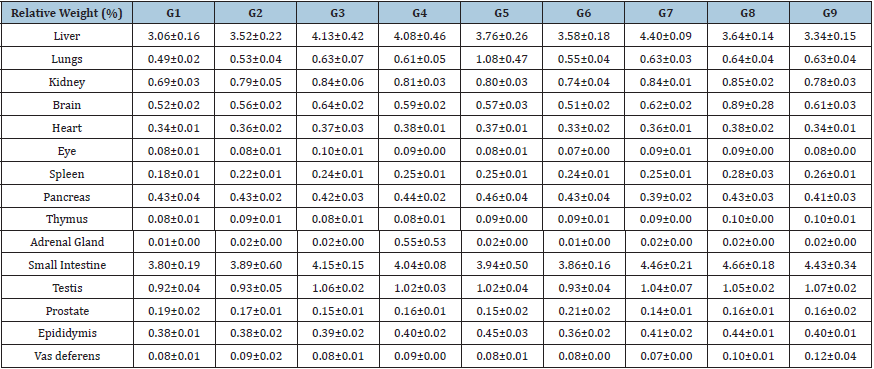
Animal weight parameters, feed intake, and relative organ weight parameters were studied and it was found that change in animal weight, daily feed intake, and organ weight were found to be normal and no significant changes were observed throughout the experimental period. Organ to body weight ratio is the valuable index for any experimental test procedure with respect to the documentation of swelling, atrophy, or hypertrophy after exposure of test samples. The results of animal tested organ weight parameters with respect to body weight are summarized in the (Table 4). Thus, the relative organ weight parameters as summarized did not found with any significant change in the organ weight throughout the experiment suggested that the test formulation was found to be safe for the treatment. Overall, the animal weight data, relative organ weight, and feed intake data suggested no significant changes were reported as compared with the untreated and disease control group (G2), it suggest that Mr. Trivedi’s Biofield Energy were found as safe in all the tested animals. This experimental, four preventive maintenance groups were used. These groups were G6, G7, G8, and G9. Results showed the significant slowdown of stress-related symptoms/complications and reduced the chances of disease susceptibility. All-inclusive, it suggests that the Biofield Energy Healing Therapy/Blessing was found to be most effective and benefited to prevent and protect from various diseases and that will ultimately improve the overall health and quality of life.
Table 4: Effect of the biofield energy treatment to the test formulation and animals per se on organ weight parameters of male Sprague Dawley rats.
Conclusion
Unpredictable Chronic Stress (UCS) animal model was used for studying the effect of oral administration of biofield energy treated/blessed test formulation for the estimation of fundamental physiological functional biomarkers. TLC count and lymphocytes were significantly increased upto maximum 82.53% and 83.86%, respectively in the G7 group as compared with the G2 group. In addition, monocyte counts were also significantly increased upto maximum 82.52% in the G5 group as compared with the G2. Besides, HDL level was significantly increased upto maximum 46.64% in the G5 group, while total cholesterol was decreased maximum by 44.29% in the G8 group as compared with the G2. However, the level of VLDL was significantly reduced by max. 44.30% in the G8 group as compared with the G2 group.
Hepatic biomarkers analysis showed that ALP and SGOT levels were decreased by 27.97% and 21.45%, respectively in the G5 group as compared with the G2. CK-MB level was significantly reduced by 37.01% in the G9 group as compared with the G2. However, animal weight parameters such as organ to body weight ratio, body weight, and feed intake data showed that the changes were nonsignificant and no toxic effect was observed after the experimental period. Therefore, the biofield energy treatment/blessing could be considered as preventive maintenance therapy in order to maintain good health and to improve quality of life. Mr. Trivedi’s Biofield Therapy could reduce the severity of disease-related to blood profile such as anaemia, bleeding disorders like haemophilia, blood clots, and blood cancers such as leukemia, lymphoma, and myeloma. In addition, the test formulation can be also used to improve various neuromuscular diseases and acute myocardial infarction. Similarly, this can be also used against autoimmune-related and inflammatory towards diseases, cancer, improve exercise capacity in heart-related disorders, Crohn’s disease, autoimmune diseases, and its various immune deficiency diseases. All-inclusive, the data encouraged that the Trivedi Effect® showed a significant humoral and cellular immune responses.
Acknowledgement
The authors are grateful to Dabur Research Foundation, Trivedi Science, Trivedi Global, Inc., and Trivedi Master Wellness for the assistance and support during the work.
References
- Dhama K, Latheef SK, Dadar M, Samad HA, Munjal A, et al. (2019) Biomarkers in stress related diseases/disorders: Diagnostic, prognostic, and therapeutic values. Front Mol Biosci 6: 91.
- Prajapati BM, Gupta JP, Pandey DP, Parmar GA, Chaudhari JD (2017) Molecular markers for resistance against infectious diseases of economic importance. Vet World 10(1): 112-120.
- Selleck MJ, Senthil M, Wall NR (2017) Making meaningful clinical use of biomarkers. Biomark Insights 12.
- Ewert A, Chang Y (2018) Levels of nature and stress response. Behav Sci 8(5): 49.
- Tampa M, Sarbu MI, Mitran MI, Mitran CI, Matei C, et al. (2018) The pathophysiological mechanisms and the quest for biomarkers in psoriasis, a stress-related skin disease. Dis Markers: 5823684.
- McEwen BS (2015) Biomarkers for assessing population and individual health and disease related to stress and adaptation. Metabolism 64(3): S2-S10.
- Marco RA, De Almeida AM, Cristobal S, Rodrigues P, Roncada P, et al. (2016) Proteomics and the search for welfare and stress biomarkers in animal production in the one-health context. Mol Biosyst 12(7): 2024-2035.
- Unato AK, Pontes WM, De Souza DMSD, Prazeres J, Marcucci LS, et al. (2018) Strength training session induces important changes on physiological, immunological, and inflammatory biomarkers. J Immunol Res: 9675216.
- Hefnawy A, Helal MAY, Sabek A, Shousha S (2018) Clinical, behavioral and biochemical alterations due to shearing stress in Ossimi sheep. J Vet Med Sci 80(8): 1281-1286.
- Chacko S, Haseeb S, Glover BM, Wallbridge D, Harper A (2018) The role of biomarkers in the diagnosis and risk stratification of acute coronary syndrome. Future Sci OA 4(1): FSO251.
- Dookhun MN, Sun Y, Zou H, Cao X, Lu X (2018) Classification of new biomarkers of dilated cardiomyopathy based on pathogenesis-an update. Health 10(3): 300-312.
- Wopereis S, Stroeve JHM, Stafleu A, Bakker GCM, Burggraaf J, et al. (2017) Multi-parameter comparison of a standardized mixed meal tolerance test in healthy and type 2 diabetic subjects: The PhenFlex challenge. Genes Nutr 12: 21.
- Karwi QG, Uddin GM, Ho KL, Lopaschuk GD (2018) Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med 5: 68.
- Marcato F, Brand H, Kemp B, Reenen K (2018) Evaluating potential biomarkers of health and performance in veal calves. Front Vet Sci 5: 133.
- Patterson ZR, Abizaid A (2013) Stress induced obesity: Lessons from rodent models of stress. Front Neurosci 7: 130.
- Nisman H, Matheson K (2005) Stress, depression, and anhedonia: Caveats concerning animal models. Neurosci Biobehav Rev 29(4-5): 525-546.
- Koithan M (2009) Introducing complementary and alternative therapies. J Nurse Pract 5(1): 18-20.
- Barnes PM, Bloom B, Nahin RL (2008) Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 12: 1-23.
- Fan KW (2005) National center for complementary and alternative medicine website. J Med Libr Assoc 93(3): 410-412.
- Wisneski L, Anderson L (2009) The scientific basis of integrative medicine. CRC Press, USA, p. 205.
- Mahendra KT, Alice B, Dahryn T, Snehasis J (2021) Effect of consciousness energy healing treatment on the metal profile and properties of tellurium. Eng Technol Open Acc 3(5): 555623.
- Mahendra KT, Alice B, Dahryn T, Snehasis J (2021) Consciousness energy healing treatment impacted the isotopic abundance ratio of 6-Mercaptopurine (6-MP). Nov Appro Drug Des Dev 5(5): 555673.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica). Journal of Food and Nutrition Sciences 3: 245-250.
- Trivedi MK, Jana S (2021) Anti-aging activity of biofield energy treated novel proprietary test formulation by assessment of vital biomarkers in Cerebrospinal Fluid (CSF) in Sprague Dawley rats. On J Neur & Br Disord 5(2).
- Trivedi MK, Jana S (2021) Evaluation of biofield energy healing treatment based proprietary test formulation on gut health potential in colon cancer cell line (HT-29). J Pharmacol Clin Res 8(4):
- Trivedi MK, Branton A, Trivedi D, Jana S (2021) Isotopic abundance ratio analysis of consciousness energy healing treated folic acid. Food Nutr Current Res 4(2): 290-295.
- Trivedi MK, Branton A, Trivedi D, Jana S (2020) The consciousness energy healing treatment and its impact on the isotopic abundance ratio analysis of flutamide. Drug Des Int Prop Int J 3(5):
- Chhabra G (2018) Automated hematology analyzers: Recent trends and applications. J Lab Physicians 10(1): 15-16.
- Drewinko B, Bollinger P, Rountree M, Johnston D, Corrigan G, et al. (1982) Eight-parameter automated hematology analyzers: Comparison of two flow cytometric systems. American Journal of Clinical Pathology 78(5): 738-747.
- Schaefer EJ, Tsunoda F, Diffenderfer M, Eliana P, Ngoc T, et al. (2016) The measurement of lipids, lipoproteins, apolipoproteins, fatty acids, and sterols, and next generation sequencing for the diagnosis and treatment of lipid disorders.
- Bodor GS (2016) Biochemical markers of myocardial damage. EJIFCC 27(2): 95-111.
- Chanif M, Chandra AP, Herlina P (2019) Preventive study garlic extract water (Allium sativum) toward SGPT, SGOT, and the description of liver histopathology on rat (Rattus norvegicus), which were exposed by Rhodamine B. IOP Conf Ser: Mater Sci Eng 546.
- Balakrishnan K, Adams LE (1995) The role of the lymphocyte in an immune response. Immunol Invest 24(1-2): 233-244.
- Bunglavan SJ, Garg AK, Dass RS, Shrivastava S (2014) Effect of supplementation of different levels of selenium as nanoparticles/sodium selenite on blood biochemical profile and humoral immunity in male wistar rats. Vet World 7(12): 1075-1081.
- Fox C, Ramsoomair D, Carter C (2001) Magnesium: Its proven and potential clinical significance. South Med J 94(12): 1195-1201.
- Payahoo L, Ostadrahimi A, Mobasseri M, Bishak YK, Farrin N, et al. (2013) Effects of zinc supplementation on the anthropometric measurements, lipid profiles and fasting blood glucose in the healthy obese adults. Adv Pharm Bull 3(1): 161-165.
- Giannini EG, Testa R, Savarino V (2005) Liver enzyme alteration: A guide for clinicians. CMAJ 172(3): 367-379.
- Sidhu P, Garg ML, Dhawan DK (2004) Protective effects of zinc on oxidative stress enzymes in liver of protein-deficient rats. Drug Chem Toxicol 19(16): 341-347.
- Boshy ME, Risha EF, Abdelhamid FM, Mubarak MS, Hadda TB (2015) Protective effects of selenium against cadmium induced hematological disturbances, immunosuppressive, oxidative stress and hepatorenal damage in rats. J Trace Elem Med Biol 29: 104-110.
- Karandish M, Tamimi M, Shayesteh AA, Haghighizadeh MH, Jalali MT (2013) The effect of magnesium supplementation and weight loss on liver enzymes in patients with nonalcoholic fatty liver disease. J Res Med Sci 18(7): 573-579.
© 2021 © Snehasis Jana. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






