- Submissions

Full Text
Significances of Bioengineering & Biosciences
Perspective on Membrane Protein Research
Indra D Sahu*
Natural Science Division, Campbellsville University, Campbellsville, USA
*Corresponding author: Indra D Sahu, Natural Science Division, Campbellsville University, Campbellsville, KY 42718, USA
Submission: February 8, 2021 Published: May 26, 2021

ISSN 2637-8078Volume5 Issue1
Abstract
Membrane proteins are proteins present in the cell membranes either spanning the width of the bilayer membrane or interacting with the surface of the membrane. They are involved in essential biological functions for the survival of living organisms. Their functions include transportation of ions and molecules across the cell membrane and initiating the signaling pathways. They are potential target of more than 60% of the modern medical drugs. Mutations or misfolding of membrane proteins are connected to numerous human dysfunctions, diseases and disorders. Structural and dynamic properties of membrane proteins are very essential to understand their function. Despite the biological importance of membrane proteins, limited information exits about these systems. Here, we briefly discuss current situation of membrane protein research including challenges and recent progress on structure biology approaches and their impact on solving pertinent biological questions related to membrane proteins.
Keywords: Membrane proteins; Biophysical techniques; Structural dynamics
Abbreviations: FDA: Food and Drug Administration; GPI: Glycosylphosphatidylinositol; PDB: Protein Data Bank; SMALPs: Styrene Maleic Acid Lipid Particles; SMA: Styrene Maleic Acid; NMR: Nuclear Magnetic Resonance; FRET: Förster Resonance Energy Transfer; Cryo-EM: Cryogenic Electron Microscopy; EPR: Electron Paramagnetic Resonance
Perspective
Membrane proteins mediate processes that are important for the function of biological cells. Membrane embedded proteins move ions and solutes across the membrane, communicate between the cell and its environment and catalyze chemical reactions [1-3]. Membrane proteins make up approximately 23% of the human proteome [4]. Membrane proteins account for more than 60% of the targets of all FDA (Food and Drug Administration) approved small molecule drugs [4]. Membrane proteins interact with membrane bilayer in various ways including the form of single pass transmembrane, multi pass transmembrane, lipid chain-anchored, Glycosylphosphatidylinositol (GPI) anchored, and membrane peripheral proteins [5]. Several human dysfunctions, diseases and disorders are linked with abnormal membrane protein functions caused by their mutations or misfolding. Detailed structural and dynamic information of membrane proteins are crucial for understanding intermolecular interactions, protein functions, and regulations [6-8]. Despite the clear biological importance of membrane proteins, detailed information about these systems are lacking [9,10]. In recent years, great efforts have been made in technological advancements in structural biology to study membrane protein structures [11]. However, the overall proportion of membrane protein entries in the Protein Data Bank (PDB) remains <4% [12]. This deficiency is due to difficulties associated with protein expression and purification, and challenges associated with the identification of lipid membrane environment that can closely mimic their native environment [8,12,13]. Understanding the function of membrane protein needs the understanding of how the protein interacts with lipid membrane environment. Membrane proteins are incorporated into lipid bilayers in numerous different ways or orientations. The helical transmembrane segment spanning the membrane bilayer width can have different lengths or it can be curved in the middle of the membrane bilayers. They may cross the membrane at different angles, or form repeated loops. The helical region of the protein may stay flat on membrane surface. The arrangement of different segment of membrane proteins may cause some portion of the protein very flexible while other highly hydrophobic during the interaction with lipid membrane [5]. The transmembrane domains of membrane protein play very active role in oligomerization and specifically drive protein-protein interactions within the plasma membrane [4]. Our current understanding of structural dynamics and functional relationship of membrane proteins is limited when compared to that of soluble proteins. The major focus of membrane protein researchers is to obtain functionally active membrane mimetic systems and appropriate biophysical techniques of structure biology to obtain higher quality of biophysical characterization data on membrane proteins. A great effort has been made in developing soluble membrane environments for membrane protein biophysical studies. However, no membrane mimetic systems are universally compatible to all membrane proteins as known up to yet, and hence it requires costly and rigorous time-consuming optimization process for incorporating membrane proteins in more native membrane environment. Biophysical studies on membrane proteins in present time utilize several membrane mimetic systems including detergent micelles, bicelles, liposomes, lipodics, lipodisq nanoparticles/SMALPs (Styrene Maleic Acid Lipid Particles) depending on types of biophysical techniques employed. These membrane mimetic systems have their own benefits and limitations. Detergent micelles are very useful for solubilizing membrane proteins outside of their native bilayer environment utilized for their biophysical characterization. However, it is very difficult to examine whether the biophysical information obtained on proteindetergent micelle system reflects its biologically functional state. Bicelles are artificial lipid bilayers obtained by mixing long chain lipid and a short chain detergent. However, it is difficult to find the universal lipid and detergent combination that can be applied to most of membrane protein systems for biophysical studies. Liposomes are used to maintain the native membrane environment for biophysical studies of membrane proteins. An illustrative example of the incorporation of an integral membrane protein Phospholamban (PLB) into lipid bilayers is shown in (Figure 1). The heterogeneous nature and larger size restrict their application to certain biophysical techniques including NMR spectroscopy [14]. It is also difficult to concentrate proteins into liposomes resulting in poor signal-to-noise in biophysical spectral measurements [11]. The another choice of membrane mimetic systems is the membrane scaffold protein based nano disc having no limitation of lipid types [15-17]. Nanodisc is recently very popular in biophysical studies of membrane proteins. However, the presence of scaffold protein may affect the optical properties of the target proteins. An emerging membrane mimetic system, known as lipodisq nanoparticles or styrene maleic acid lipid nanoparticles (SMALPs) has been rapidly gaining popularity in enhancing the biophysical studies of membrane proteins [13,18-30]. Lipodisq nanoparticles are formed by combining Styrene Maleic Acid (SMA) copolymer and phospholipids in detergent free environment which can maintain structural and functional properties of membrane proteins which is very challenging for traditional membrane mimetics. Lipodisq nanoparticles are appropriate for several biophysical approaches including NMR and EPR spectroscopic techniques [31,32].
Figure 1: Cartoon representation of an illustrative example of a membrane Protein Phospholamban (PLB) (PDB ID:1FJK) [31] incorporated into lipid bilayers (POPC/POPG). Image was prepared using Visual Molecular Dynamics (VMD) [32] and molecular modeling was performed using CHARMMGUI (www.charmm-gui.org).
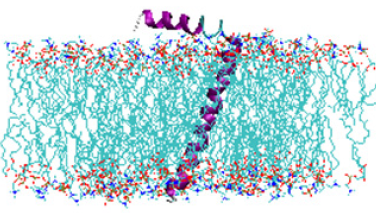
In recent years, tremendous improvements have been made in technical and methodological aspects of structural biology approaches for studying membrane proteins. Due to challenges associated with membrane protein sample preparation in functionally active lipid membrane environment, application of biophysical methods are limited for membrane protein studies [30,33-38]. Some of the popular biophysical techniques used to study membrane proteins include X-ray crystallography, Nuclear Magnetic Resonance (NMR) spectroscopy, Förster Resonance Energy Transfer (FRET), Cryogenic Electron Microscopy (Cryo-EM), and Electron Paramagnetic Resonance (EPR) spectroscopy. These biophysical techniques have their own advantages and limitations. X-ray crystallographic technique is used to obtain high quality three-dimensional structural data on membrane proteins but cannot provide information on their dynamic properties [39,40]. The challenges introduced in crystallization of membrane proteins also limit the application of this technique to many membrane proteins [41]. NMR spectroscopy is used to probe structural and dynamic properties of membrane proteins in a physiological condition, but this technique is limited by the larger size of proteinmembrane complex (>~50 kD) [41-44]. Additional challenges using NMR techniques for membrane protein studies are caused by the wider spectral linewidth and spectral overlapping, and the requirement of a large amount of highly pure and properly folded membrane protein samples to obtain high resolution structural data [33,37,45,46]. Another technique for studying conformational changes of membrane proteins system is a probe based Förster Resonance Energy Transfer (FRET) technique [47]. The use of larger probe size in this technique may induce higher structural perturbation. Also, the incorporation of the FRET probe at the specific site of the membrane protein sequence is very challenging [48]. Cryogenic Electron Microscopy (Cryo-EM) has been rapidly growing as a powerful structural biology tool for probing threedimensional structure of membrane proteins at near-atomic resolutions [49,50]. Cryo-EM technique needs very small amount of samples with no requirement of protein crystallization removing limitations of X-ray crystallography and NMR spectroscopy [49]. The resolution of this technique is significantly low for the lower size membrane proteins (<~50 kDa) limiting its application to many membrane proteins [51]. A recent example of using Cryo-EM is a study of Escherichia coli lipid transporter MsbA and Escherichia coli mechanosensitive channel MscS reconstituted into peptidiscs [52]. Angiulli et al. [52] utilized Cryo-EM microscopy to obtain a 4.2Å resolution structure of MsbA reconstituted into peptidisc that revealed that the peptidisc preserves the native conformation of MsbA as well as its interaction with its Lipopolysaccharide (LPS) cargo [52]. Similarly, a 3.3Å ̊ resolution structure of the homoheptameric mechanosensitive channel MscS reconstituted into peptidisc was also determined [52]. Their results suggested the arrangements of the peptidisc peptides around MsbA and MscS are very different. This study further revealed the structural basis for how the pseptidisc scaffold can adapt to membrane proteins of different sizes, shapes and symmetries. Electron Paramagnetic Resonance (EPR) spectroscopy has been emerged as a rapidly expanding powerful structural biology tool to overcome these limitations and provide prominent structural and dynamic information on membrane proteins [7,8,30,48,53-55]. EPR spectroscopy in association with Site-Directed Spin Labeling (SDSL) can provide structural dynamics of nitroxide side-chain, solvent accessibility, solvent polarity, and intra- or intermolecular distances between two nitroxide spin labels on membrane proteins [7,8,30,56,57]. The flexible nature of the spin label used in EPR spectroscopy has made possible to incorporate spin labels at nearly any specific site of protein sequence without any size restriction. Despite the great benefit of SDSL EPR spectroscopy over other biophysical techniques, a care should be taken while selecting spin labeling sites for the study, because the incorporation of certain kind of spin labels may cause structural and functional perturbations. A recent example of using EPR spectroscopy is the investigation of a biologically important membrane protein Pinholin S21 (a class-II holin, encoded by the S21 gene of phage Φ21) in DMPC (1,2-dimyristoyl-snglycero-3-phosphocholine) proteoliposomes [58]. Ahmmad et al. [58] utilized five sets of inter label DEER distances measured between transmembrane domains 1 and 2 (TMD1 and TMD2) of both the active and inactive forms of pinholin S21 using the four pulse DEER technique of EPR spectroscopy as experimental DEER distance restraints in combination with the simulated annealing software package Xplor- NIH to predict structural models of the active pinholin (S2168) and inactive antipinholin (S2168IRS).
Conclusion
In conclusion, membrane proteins are very important biological systems essential for the survival of living organisms. Despite the challenges associated with sample preparation in functionally relevant native membrane environment, a tremendous progress has been made in developing membrane environments compatible with biophysical approaches for studying membrane proteins. The technical and methodological advances in the biophysical approaches enable researchers to answer pertinent biological questions associated with several important complicated membrane protein systems. Although great achievements have been made recently, several research areas of membrane proteins still need to be taken attention, such as technical and methodological developments in improving the bacterial expression yield of the membrane protein, stabilization and sulubilization of membrane proteins in more native like environments.
Acknowledgement
I would like to acknowledge support from an NSF MCB- 2040917 award.
References
- Cournia Z, Allen TW, Andricioaei I, Antonny B, Baum D, et al. (2015) Membrane protein structure, function, and dynamics: A perspective from experiments and theory. Journal of Membrane Biology 248(4): 611-640.
- Congreve M, Marshall F (2010) The impact of GPCR structures on pharmacology and structure-based drug design. Br J Pharmacol 159(5): 986-996.
- Baker M (2010) Structural biology: The gatekeepers revealed. Nature 465: 823-826.
- Yin H, Flynn AD (2016) Drugging membrane protein interactions. Annual Review of Biomedical Engineering 18(18): 51-76.
- Chou KC, Elrod DW (1999) Prediction of membrane protein types and subcellular locations. Proteins-Structure Function and Genetics 34(1): 137-153.
- Engel A, Gaub HE (2008) Structure and mechanics of membrane proteins. Annual Review of Biochemistry 77: 127-148.
- Klug CS, Feix JB (2008) Methods and applications of site-directed spin labeling EPR spectroscopy. Methods Cell Biol 84: 617-658.
- Sahu ID, McCarrick RM, Lorigan GA (2013) Use of electron paramagnetic resonance to solve biochemical problems. Biochemistry 52(35): 5967-5984.
- Das BB, Park SH, Opella SJ (2015) Membrane protein structure from rotational diffusion. Biochim Biophys Acta 1848(1): 229-245.
- Kang HJ, Lee C, Drew D (2013) Breaking the barriers in membrane protein crystallography. Int J Biochem Cell Biol 45(3): 636-644.
- Carpenter EP, Beis K, Cameron AD, Iwata S (2008) Overcoming the challenges of membrane protein crystallography. Current Opinion in Structural Biology 18(5): 581-586.
- Puthenveetil R, Vinogradova O (2019) Solution NMR: A powerful tool for structural and functional studies of membrane proteins in reconstituted environments. Journal of Biological Chemistry 294(44): 15914-15931.
- Sahu ID, MaCarrick RM, Troxel KR, Zhang R, Smith JH, et al. (2013) DEER EPR measurement for membrane protein structures via bifunctional spin labels and lipodisq nanoparticles. Biochemistry 52(38): 6627-6632.
- Hemminga MA, Berliner LJ (2007) ESR spectroscopy in membrane biophysics. (1st edn), Springer Publishers, USA, pp. 1-341.
- Bayburt TH, Sligar SG (2010) Membrane protein assembly into nanodiscs. Febs Letters 584(9): 1721-1727.
- Bayburt TH, Sligar SG (2003) Self-assembly of single integral membrane proteins into soluble nanoscale phospholipid bilayers. Protein Science 12(11): 2476-2481.
- Denisov IG, Grinkova YV, Lazarides AA, Sligar SG (2004) Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. Journal of the American Chemical Society 126(11): 3477-3487.
- Orwick Rydmark M, Lovett JE, Graziadei A, Lindholm L, Hicks MR, et al. (2012) Detergent-free incorporation of a seven-transmembrane receptor protein into nanosized bilayer lipodisq particles for functional and biophysical studies. Nano Letters 12(9): 4687-4692.
- Jamshad M, Lin YP, Knowles TJ, Parslow RA, Harris C, et al. (2011) Surfactant-free purification of membrane proteins with intact native membrane environment. Biochemical Society Transactions 39(3): 813-818.
- Rajesh S, Knowles T, Overduin M (2011) Production of membrane proteins without cells or detergents. New Biotechnology 28(3): 250-254.
- Long AR, O Brien CC, Malhotra K, Schwall CT, Albert AD, et al. (2013) A detergent-free strategy for the reconstitution of active enzyme complexes from native biological membranes into nanoscale discs. BMC Biotechnology 13: 41.
- Jamshad M, Grimard V, Idini I, Knowles TJ, Dowle MR, N, et al. (2015) Structural analysis of a nanoparticle containing a lipid bilayer used for detergent-free extraction of membrane proteins. Nano Research 8(3): 774-789.
- Lund A, Andersson P, Eriksson J, Hallin J, Johansson T, et al. (2008) Automatic fitting procedures for EPR spectra of disordered systems: Matrix diagonalization and perturbation methods applied to fluorocarbon radicals. Spectrochimica Acta Part A 69(5): 1294-1300.
- Zhang R, Sahu ID, Liu L, Osatuke A, Corner RG, et al. (2015) Characterizing the structure of lipodisq nanoparticles for membrane protein spectroscopic studies. Biochimica Et Biophysica Acta-Biomembranes 1848(1): 329-333.
- Kim SS, Upshur MA, Saotome K, Sahu ID, McCarrick RM, et al. (2015) Cholesterol-dependent conformational exchange of the c-terminal domain of the influenza A M2 Biochemistry 54(49): 7157-7167.
- Dorr JM, Scheidelaar S, Koorengevel MC, Dominguez JJ, Schafer M, et al. (2016) The styrene-maleic acid copolymer: A versatile tool in membrane research. European Biophysics Journal 45(1): 3-21.
- Craig AF, Clark EE, Sahu ID, Zhang R, Frantz ND, et al. (2016) Tuning the size of styrene-maleic acid copolymer-lipid nanoparticles (SMALPs) using RAFT polymerization for biophysical studies. Biochimica Et Biophysica Acta 1858(11): 2931-2939.
- Dorr JM, Koorengevel MC, Schafer M, Prokofyev AV, Scheidelaar S, et al. (2014) Detergent-free isolation, characterization, and functional reconstitution of a tetrameric K+ channel: The power of native nanodiscs. Proceedings of the National Academy of Sciences of the United States of America 111(52): 18607-18612.
- Sahu ID, Kroncke BM, Zhang R, Dunagan MM, Smith HJ, et al. (2014) Structural investigation of the transmembrane domain of KCNE1 in proteoliposomes. Biochemistry 53(40): 6392-6401.
- Sahu ID, Lorigan GA (2020) Electron paramagnetic resonance as a tool for studying membrane proteins. Biomolecules 10(5): 763.
- Lamberth S, Schmid H, Muenchbach M, Vorherr T, Krebs J, et al. (2000) NMR solution structure of phospholamban. Helvetica Chimica Acta 83(9): 2141-2152.
- Humphrey W, Dalke A, Schulten K (1996) VMD-visual molecular dynamics. J Molec Graphics 14(1): 33-38.
- Liang B, Tamm LK (2016) NMR as a tool to investigate membrane protein structure. Dynamics and function of membrane proteins. Nat Struct Mol Biol 23(6): 468-474.
- Bordag N, Keller S (2010) Alpha-helical transmembrane peptides: A "Divide and Conquer" approach to membrane proteins. Chemistry and Physics of Lipids 163(1): 1-26.
- Moraes LGM, Fazio MA, Vieira RFF, Nakaie CR, Miranda MTM, et al. (2007) Conformational and functional studies of gomesin analogues by CD, EPR and fluorescence spectroscopies. Biochimica Et Biophysica Acta-Biomembranes 1768(1): 52-58.
- Baker M (2010) Making membrane proteins for structures: A trillion tiny tweaks. Nat Meth 7(6): 429-434.
- Torres J, Stevens TJ, Samso M (2003) Membrane proteins: the 'Wild West' of structural biology. Trends Biochem Sci 28(3): 137-144.
- Huang C, Mohanty S (2010) Challenging the limit: NMR assignment of a 31 kDa helical membrane protein. J Am Chem Soc 132(11): 3662-3663.
- Columbus L, Hubbell WL (2002) A new spin on protein dynamics. Trends Biochem Sci 27(6): 288-295.
- Kermani AA (2020) A guide to membrane protein X-ray crystallography. The FEBS Journal pp. 1-17.
- Acharya KR, Lloyd MD (2005) The advantages and limitations of protein crystal structures. Trends Pharmacol Sci 26(1): 10-14.
- Wuthrich K (2003) NMR studies of structure and function of biological macromolecules (Nobel Lecture). J Biomol NMR 27(1): 13-39.
- Klare JP (2012) Site-directed spin labeling and electron paramagnetic resonance (EPR) spectroscopy: A versatile tool to study protein-protein interactions. In Tech, pp. 1-22.
- Schiemann O, Prisner TF (2007) Long-range distance determinations in biomacromolecules by EPR spectroscopy. Quarterly Rev Biophys 40(1): 1-53.
- Midgett CR, Madden DR (2007) Breaking the bottleneck: Eukaryotic membrane protein expression for high-resolution structural studies. Journal of Structural Biology 160(3): 265-274.
- Bahar I, Lezon TR, Bakan A, Shrivastava IH (2010) Normal mode analysis of biomolecular structures: Functional mechanisms of membrane proteins. Chemical Reviews 110(3): 1463-1497.
- Loura LMS, Prieto M (2011) FRET in membrane biophysics: An overview. Front Physiol 2: 82.
- Claxton DP, Kazmier K, Mishra S, McHaourab HS (2015) Navigating membrane protein structure, dynamics, and energy landscapes using spin labeling and EPR spectroscopy. Methods Enzymol 564: 349-387.
- Callaway E (2020) The protein-imaging technique taking over structural biology. Nature 578: 201-201.
- Autzen HE, Julius D, Cheng YF (2019) Membrane mimetic systems in CryoEM: Keeping membrane proteins in their native environment. Current Opinion in Structural Biology 58: 259-268.
- Liu YX, Huynh DT, Yeates TO (2019) A 3.8 angstrom resolution cryo-EM structure of a small protein bound to an imaging scaffold. Nature Communications 10.
- Angiulli G, Dhupar HS, Suzuki H, Wason IS, Van Hoa FD, et al. (2020) New approach for membrane protein reconstitution into peptidiscs and basis for their adaptability to different proteins. Elife 9.
- Fanucci GE, Cafiso DS (2006) Recent advances and applications of site-directed spin labeling. Curr Opinion Struct Biol 16(5): 644-653.
- Hubbell WL, McHaourab HS, Altenbach C, Lietzow MA (1996) Watching proteins move using site-directed spin labeling. Structure 4(7): 779-783.
- Hubbell WL, Lopez CJ, Altenbach C, Yang ZY (2013) Technological advances in site-directed spin labeling of proteins. Current Opinion in Structural Biology 23(5): 725-733.
- Sahu ID, Lorigan GA (2015) Biophysical EPR studies applied to membrane proteins. J Phys Chem Biophys 5(6): 188.
- Roser P, Schmidt MJ, Drescher M, Summerer D (2016) Site-directed spin labeling of proteins for distance measurements in vitro and in cells. Organic & Biomolecular Chemistry 14(24): 5468-5476.
- Ahammad TA, Drew DL, Sahu ID, Khan RH, Butcher BJ, et al. (2020) Conformational differences are observed for the active and inactive forms of pinholin S21 using DEER spectroscopy. Physical Chemistry B 124(50): 11396-11405.
© 2021 © Indra D Sahu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






