- Submissions

Full Text
Significances of Bioengineering & Biosciences
Evaluation of the Antibacterial Activities of Some Newly Prepared Schiff Bases and Schiff- Based Resins Against Staphylococcus Aureus and Escherichia Coliy
Nasira Liaquat Rajput1*, Moina Akhtar Moghal1, Khalid Mohammed Khan2, Aamna Balouch3, Sarfraz Ali Tunio4, Iqra Shahid Arain1 and Samra Qaimkhani1
1M A Kazi Institute of Chemistry University of Sindh, Pakistan
2H E J Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Pakistan
3Istanbul Technical University, Faculty of Science and Letters, Department of Physics Engineering, Turkey
4Institute of Microbiology Department University of Sindh Jamshoro, Pakistan
*Corresponding author: Nasira Liaquat Rajput, M A Kazi Institute of Chemistry University of Sindh, Jamshoro-76080, Pakistan
Submission: February 25, 2021 Published: April 2, 2021

ISSN 2637-8078Volume4 Issue5
Abstract
The present study examines the synthesis and antibacterial activities of two newly prepared Schiff bases, bis (2-hydroxy-1-napthaldehyde hydrazine hydrate) BHNHH and bis(2-hydroxy-1-napthaldehyde m-xylene diimine) and two newly synthesized Schiff based-resins, polymethylene bis (2-hydroxy-1-napthaldehyde hydrazine hydrate) PMBHNHH and polymethylene bis(2-hydroxy-1-napthaldehyde m-xylene diimine) PMBHNMXD. All the compounds were characterized by different spectroscopic techniques and clearly determined the structure and molecular weight of compounds. The synthesized compounds were successfully used for antibacterial activity against Gram-positive and Gram-negative bacterial strains because the prepared Schiff bases and resins contain biologically active azomethine functional group, thus, the antibacterial activity was screened against Staphylococcus Aureus (Gram-positive) and Escherichia Coli (Gram-negative) by agar well diffusion method. The data confirmed that the synthesized compounds were highly active against both Gram-positive and Gram-negative bacterial strains. The broad-spectrum antibacterial activity against the reported bacteria may be due to the delocalizing of pi electrons of entering the cell wall of test bacteria, which may result in an enhancement in the lipophilic side of compounds and killing them. In summary, Schiff bases bis (2-hydroxy-1-napthaldehyde hydrazine hydrate) BHNHH and bis(2-hydroxy-1-napthaldehyde m-xylene diimine) and Schiff based resins polymethylene bis(2-hydroxy-1-napthaldehyde hydrazine hydrate) PMBHNHH and polymethylene bis(2-hydroxy-1-napthaldehyde m-xylene diimine) PMBHNMXD possessed potent antibacterial activity against Gram-positive and Gram-negative bacterial strains and may be explored for their potential therapeutic purposes.
Keywords: Antimicrobial; Protozoan; Escherichia coli; Microorganism; Gram-positive; Bacterial strains
Introduction
Results
Schiff base polymers containing conjugated carbon, carbon double bonds of carbonnitrogen in their chain have been in consideration of researchers due to their significance in many aspects [1,2]. Schiff bases are the important intermediate for the synthesis of various bioactive compounds. Furthermore, they are reported to exhibit a variety of biological activities [3,4]. A number of tests regarding antibacterial activities have been conducted using the complexes of Schiff bases against various organisms and presented docile to average activity [5,6]. Schiff bases and their metal complexes have recently gained considerable importance due to their chelating ability of resin has been found to be more selective by nature, and have poor solubility in organic solvents and high melting point [7-9]. Schiff base resin has at all times attracted the researchers due to their vast applications in diverse fields, such as medicine, anti-amoebic agents, biological sciences and as well as in the synthesis of drugs [10,11]. To overcome the startling problem of microbial resistance to antibiotics, the quest of novel active compounds against new targets is a matter of paramount importance [12,13]. Several studies have described Schiff base polymers possessing a number of anti- microbial activities including anti-bacterial, anti-fungal, anticancer, antioxidant, anti-tumor and herbicidal activities [14-16]. Its complexes have a wide range of applications in biological and clinical fields. Antibacterial activities are independent of the molecular structure of compounds [9,17,18]. Schiff bases are connected to the existence of the intermolecular hydrogen bond and proton transfer equilibrium. This group of compounds has been characterized by great biological activity and play an important part in numerous biological systems and for their new topological behavior and unlimited area of synthetic organic chemistry [19- 21].Due to such important biological activities, organic chemists are interested to synthesize Schiff bases [22]. Antibiotic properties are used in metal transport through membrane or to attach the antibiotics to particular position from which it can interfere the growth of bacteria [23]. General use of antibacterial drugs and their resistance against bacterial infections has controlled to assist health problems [24]. Metal ion bonded to biologically active compounds may improve their biological activities. Many complexes of Schiff bases are largely studied due to synthetic flexibility, selectivity, and sensitivity toward a diversity of organisms. This would be safe in conduct of infections caused by any of these specific strains [21-23]. The aim of present study was to assess the antibacterial activities of newly prepared Schiff bases and Schiff based resins against Grampositive and Gram-negative bacterial strains.
Materials and Methods
All the starting material such as 2-Hydroxy-1-naphthaldehyde, hydrazine hydrate, and m-xylene diamine was purchased from (alfa aesar, Germany). The bacterial strains Escherichia coli (ATCC-25922), Staphylococcus aureus (ATCC-6538) used in antibacterial assay were provided by the Institute of Microbiology, University of Sindh Jamshoro. All the glassware like Petri plates were sterilized in Autoclave at 121 °C for 15 minutes and the organic solvents were refined as described in elsewhere.
Chemistry
The overall synthesis of two Schiff bases and their Schiff based resins comprise of two steps, in the first step of Schiff bases, bis (2-hydroxy-1-napthaldehyde hydrazine hydrate) BHNHH and bis(2-hydroxy-1-napthaldehyde m-xylene diimine) was synthesized while in the second step, its two Schiff based-resins, polymethylene bis(2-hydroxy-1-napthaldehyde hydrazine hydrate) PMBHNHH and polymethylene bis(2-hydroxy-1-napthaldehyde m-xylene diimine) PMBHNMXD were synthesized. The synthetic procedure of synthesized materials is reported [24]. All these newly synthesized compounds were tested for their antibacterial potential by agar well diffusion method against Escherichia coli and Staphylococcus aureus (Figures 1-3).
Figure 1(a): Reaction scheme of schiff base (BHNHH).

Figure 1(b): The problems of air pollution and possible photocatalytic solutions based on TiO2.
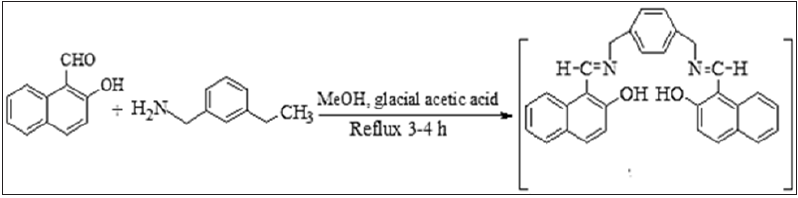
Figure 2(a):The problems of air pollution and possible photocatalytic solutions based on TiO2.
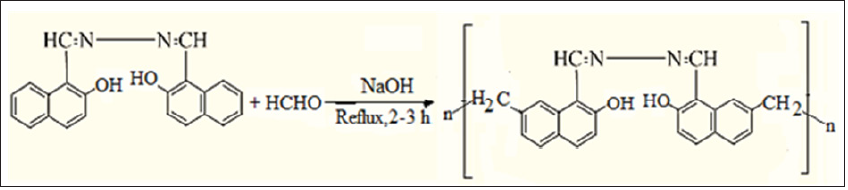
Figure 2(b): Reaction Scheme of Schiff Based Resin (PMBHNMXD).
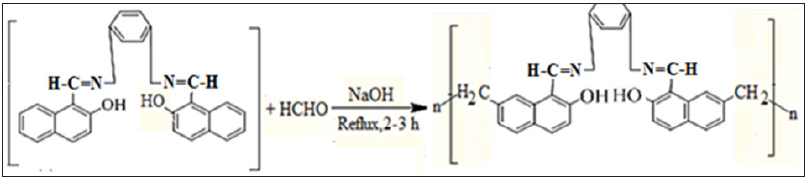
Figure 3:Graphical abstract of antibacterial activities.
Discussion
Evaluation of in vitro antibacterial activity
Schiff based compounds play a vital role as bi-or tridentate ligands producing strong complexes with transition metals. Imine complexes possess a wide range of biological properties of Schiff base and resins. The Schiff bases (BHNHH), (BHNMXD), and Schiff based resins (PMBHNHH) and (PMBHNMXD) were screened for their antibacterial potential by using agar well diffusion method. Briefly, the overnight culture of Staphylococcus aureus and Escherichia coli was used to prepare a lawn on the surface of Muller Hinton Agar plates with the help of sterile swab. The plates were left to dry and then with the help of steel borer a well of 6mm dug in the media. Then 100μL/mL i.e., 1mg mL solution of Schiff bases and solution of resins prepared in Di-Methyl Sulfoxide (DMSO) was poured into the well with the help of micropipette. One well was pored with 100μL of DMSO as control the plates were incubated at 37 ℃ for 24 hours [25]. Following day, the zones of growth inhibition were measured, and results were recorded. The zones of inhibition was measured exhibiting inhibition tetracycline was used as a standard drug for antibacterial activity.
Results and Discussion
Exploring the antibacterial potential of newly prepared Schiff bases and their respective resins
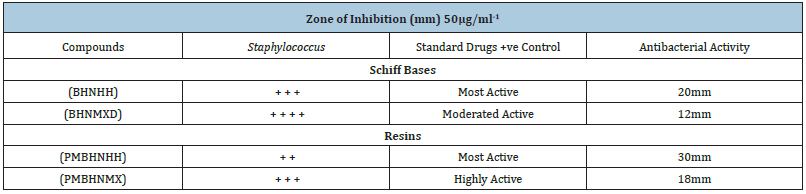
Two newly synthesized Schiff bases and two new Schiff based resins were synthesized by polycondensation reactions. These compounds and their complexes needed a range of applications including clinical, pharmacological and industrial. The compound’s antibacterial activity is associated with the structure of the cell wall of bacteria, which is extremely important for the survival of the bacterial cell. The findings of antibacterial assays revealed that the Schiff bases possessed enhanced antibacterial activities against both Gram-positive and Gram-negative bacterial strains including Staphylococcus aureus and Escherichia coli, thus considered as board spectrum compounds. The results of the Schiff bases (BHNHH), (BHNMXD), are shown in (Figure 4) (22,13mm) Zones of inhibition against the reported bacteria strain Escherichia coli exhibiting highly active and most active bacterial activity (Table 1). Whereas the resins (PMBHNHH), (PMBHNMXD) showed in (Figure 5) (25, 21mm), zones of inhibition against the bacterial strain, exhibiting moderately and highly active bacterial studies. These compounds were screen against Staphylococcus aureus and Escherichia coli. The results conveyed that the Schiff base (BHNHH), (BHNMXD), showed in (Figure 6) and (20,12mm) zones of the inhibition against the reported bacteria strain Staphylococcus aureus (G+ve) exhibiting highly active and most active bacterial activity. Where the resins (PMBHNHH),(PMBHNMXD), showed in (Figure 7) (30,18mm), zones of inhibiting in against the bacterial strain, showing moderately and highly active bacterial studies. All compounds presented enhanced activities for these strains and found strongly active against the reported bacteria this was due to the delocalizing pi electrons rupturing the bacterial wall of protozoan. This resulted in an enhancement in the lipophilic side of compounds killing them completely (Table 2).
Figure 4:The zones of inhibition of bacterial growth around the wells represent the antibacterial activities of schiff bases and resins against Escherichia coli.
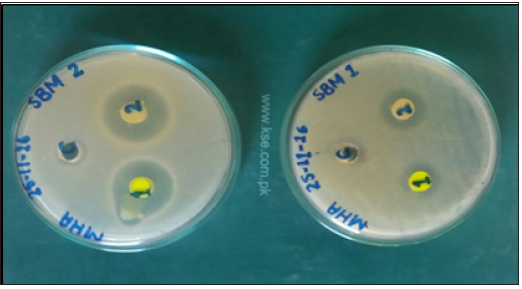
Figure 5: The zones of inhibition of bacterial growth around the wells represent the antibacterial activities of schiff bases and resins against Escherichia coli.
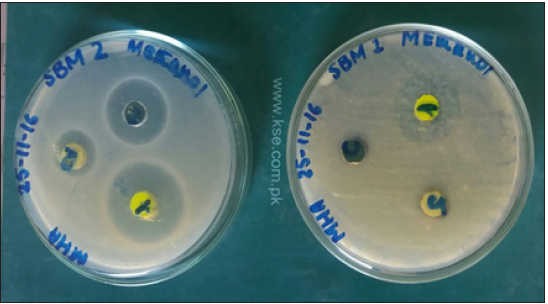
Figure 6:Bar graph representation antibacterial activities of Schiff bases and Schiff based resins Escherichia coli (G-ve).
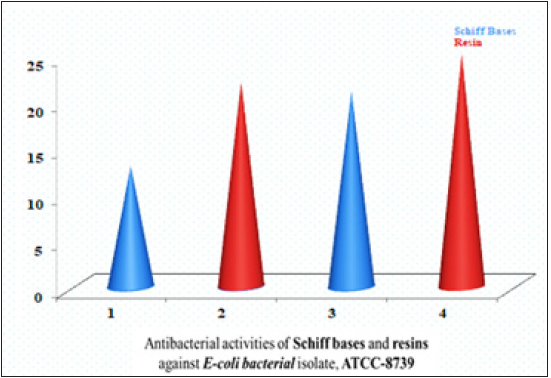
Figure 7: Bar graph representation antibacterial activities of schiff bases and schiff based resins staphylococcus aureus (G+ve).
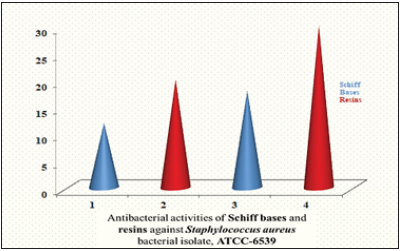
Table 1: The effect of schiff bases and resins on Escherichia coli (G-ve) bacterial growth.
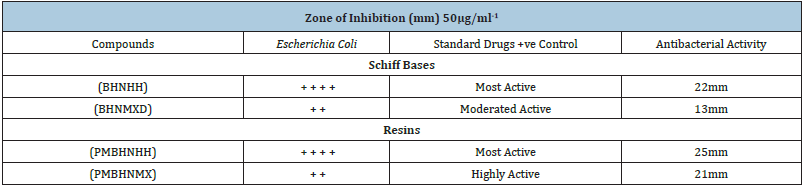
Table 2: The effect of schiff bases and resins on Staphylococcus aureus (G+ve) bacterial growth. −=Inactive. +=weakly active. ++=moderately active. +++=highly active. ++++=most active.
Conclusion
Two new Schiff bases and two Schiff base resins have been synthesized by poly condensation reactions. These compounds and their complexes needed a range of applications including clinical, pharmacological and industrial. As described in literature the compounds antibacterial activity is related to structure of cell wall of bacteria. The cell wall is extremely important for survival of the bacterial growth. In summary, all newly prepared Schiff bases and Schiff based resin contain biological active azomethine functional group, therefore, they exhibited strong antibacterial activities against Staphylococcus aureus and Escherichia coli suggesting that these compounds may be used in preparations of new antibacterial drugs. The Schiff base (BHNHH) and (BHNMXD) and respective Schiff based resins (PMBHNHH) and (PMBHNMXD) were found to display broad spectrum antibacterial activity against both Gram-negative ad Gram-positive bacterial strains. These compounds may be used as new antibacterial drugs after performing further research works with advanced technology.
The authors are grateful to Dr. M. A. Kazi Institute of Chemistry, Sindh University of Jamshoro, Pakistan, and H. E. J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi for providing the space and research facilities at Organic chemistry laboratory of both institutes. Authors are thankful to the Institute of Microbiology, University of Sindh Jamshoro, Pakistan, and Istanbul Technical University, Faculty of Science and Letters, Department of Physics Engineering, Maslak, Istanbul, Turkey.
References
- Khuhawar M, Shah A, Mughal M (2007) Preparation and characterization of schiff base polymers derived from 4, 4’-Methylene bis (Cinnamaldehyde). Chinese J Polymer Scienc 25(4): 399-407.
- Khuhawar M, Moina A, Channar AH (2004) Synthesis and characterization of some new schiff base polymers. European Polymer Journal 40(4): 805-809.
- Arulmurugan S, Kavitha H, Venkatrama BR (2010) Biological activities of schiff base and its complexes. Rasayan J Chem 3(3): 385-410.
- Moina A, Hussain A, Zeenat M, Zuhra G, Khuhawar M, et al. (2014) Preparation, characterization viscosity and thermal analysis of a schiff-based resin derived from 2-methoxy-1-napthaldehyde. Journal of Engineering 4(2): 20-27.
- Khan F, Syed F, Ali K, Wasim A, Zia ul Haq K, et al. (2015) Synthesis, spectral characterization and antibacterial study of Schiff base metal complexes derived from N-[(E)-(5-Chloro-2-Hydroxyphenyl)Methylidene]-4-Nitrobenzenesulfonamide. American Eurasian J Agric & Environ Sci 15(2): 216-219.
- Ravi Kumar AR, Reddy KH (2003) Heavy metal ion uptake properties of polystyrene-supported chelating polymer-resins. Journal of Chemical Sciences 115: 155-160.
- Moina M, Mughal A, Zuhara G, Khuhawar MY (2013) Synthesis and characterization of antimicrobial metal based polymeric ligand derived from 4,4′-methylene-bis-(furfuraldehyde). Asian Journal of Chemistry 25(11): 6327-6333.
- Moina M, Hussain A, Zuhara G, Khuhawar M, Qureshi S (2013) Synthesis, characterization, viscosity and thermos-analytical studies of schiff base polymers derived from 6, 6-methylene bis-(1-nephthaldehyde) MBN. Asian Journal of Chemistry 25.
- Mughal MA, Mughal A (2013) Antimicrobial, thermoanalytical and viscometric studies of metal based Schiff base polymer. J Chem Soc Pak 35(6): 1535-1542.
- Rathish N, Shah A, Baluja S, Chandai S (2006) Synthesis and antibacterial activity of some Schiff base complexes. J Serb Chem Soc 71(7): 733-744.
- Moina A, Hussain A, Zeenat M, Ghulam ZM, Mohammad YK, et al. (2013) New antimicrobial schiff base, polymers derived from 6,6-methylenebis (1-napthaldehyde). International Journal of Scientific & Engineering Research 4(9):120-128.
- Kumar A, Fernandes J, Kumar P (2014) Synthesis, antimicrobial and anti-inflammatory studies of some novel schiff base derivatives. Int J Drug Dev & Res 6(2): 165-171.
- Al Mosawi SK, Al Shamkhani ZA (2016) New heterocyclic Schiff base and azetidinone as antibacterial agents. International Journal of Environmental Chemistry and Ecotoxicology Research 1(3): 33-37.
- Kumar S, Sheriff K (2015) Synthesis characterization, antibacterial activity and DNA cleavage studies of Schiff base Co(II) transition metal complexes. Der Pherma Chemica 7: 79-89.
- Ibrahim M, Sharif S, EL Tajory AN, Elamari AA (2011) Synthesis and antibacterial activities of some schiff bases. E-Journal of Chemistry 8(1): 212-216.
- Yan X, Sun W (2010) Synthesis and metal ion adsorption studies of chelating resins derived from microporous glycidyl methacrylate-divinyl benzene anchored schiff base copolymer beads anchored schiff bases. Journal of Applied Polymer Science 117(2): 953-959.
- Sahu R, Thakur D, Kashyap P (2012) Schiff base: An overview of its medicinal chemistry potential for new drug molecules. International Journal of Pharmaceutical Science and Nanotechnology 5(3):1757-1764.
- Shalin K, Durga N, Saxena P (2009) Applications of metal complexes of schiff bases-A review. Journal of Scientific & Industrial Research 68: 181-187.
- Aneela W, Syed SH, Iffat M (2014) Synthesis of Schiff bases from natural product and their remarkable antimicrobial and antioxidant activity. Fuust J Bio 4(1): 27-32.
- Sonnekar Vs, Jadhav WN, Dake DS, Pawar R (2013) Synthesis and antimicrobial and antifungal activities of novel bis-imine derivatives. Research Journal of Biological and Chemical Sciences 4(2): 1411-1418.
- Islam M, Shahriar S, Islam M, Jesmin M, Ali M, et al. (2013) Antibacterial activities of some transition metal schiff base complexes. International Letter of Chemistry, Physics Astronomy 10: 12-20.
- Suleiman GN, Hassandarvish P (2014) Synthesis, characterization and anticancer studies of some morpholine derived schiff bases and their metal complexes. Journal of Applied Pharmaceutical Science 4(10): 75-80.
- Shukla S, Gaur P, Gupta J, Methews S, Rai N (2015) Synthesis characterization and anti-bacterial activity of complexes of palladium with schiff bases derived from 1,3-diaminopropane. International Journal of Basic and Applied Chemical Sciences 5(1): 18-28.
- Nasira LR, Moina AM, Aamna B, Khalid MK, Moghal AH, et al. (2019) Synthesis, crystallographic, monoclinic, inflexible, and antifungal assessment of schiff based resin. Sindh Univ Res Jour.
- Ahmed M, Ibrahim M (2015) A review on versatile applications of transition metal complexes incorporating schiff bases. Journal of Basic and Applied Science 4(2): 119-133.
© 2021 © Nasira Liaquat Rajput. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






