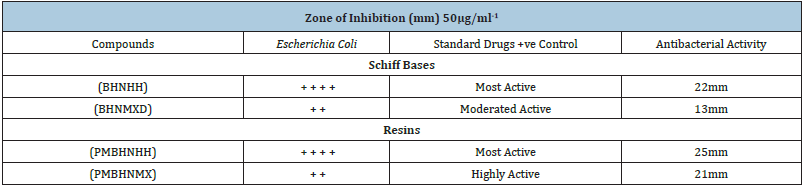
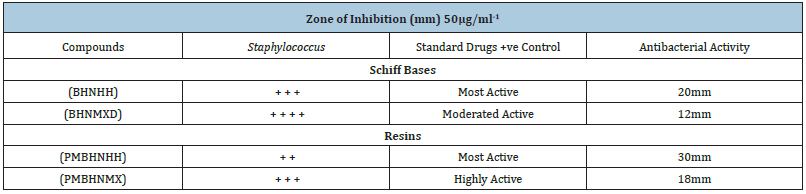
- Submissions

Full Text
Significances of Bioengineering & Biosciences
AGR2 and AKR1B10: Potential Collaborative Markers for Nasopharyngeal Carcinoma Diagnosis and Therapy
Yuan Tan1, Renhua Fan2, Liguang Lin2, Fang Liu2, Meihua Zhou3 and Lei Wang2*
1Hunan Key Laboratory of Oncotarget gene and Clinical Laboratory, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University & Hunan Cancer Hospital, China
2Department of Clinical Laboratory, Changsha Central Hospital, China
3Changsha KingMed Center for Clinical Laboratory, China.
*Corresponding author: Lei Wang, Department of Clinical Laboratory, Changsha Central Hospital, Changsha 410004, China
Submission: March 8, 2021 Published: March 26, 2021

ISSN 2637-8078Volume4 Issue4
Abstract
Nasopharyngeal Carcinoma (NPC) is a common malignant cancer in South China. Previous studies have showed that Anterior Gradient Protein 2 (AGR2) and Aldo-Keto Reductase 1B10 (AKR1B10) progressively increase in NPC primary and metastatic tissues, and AGR2 and AKR1B10 may have the potential to reflect the development of NPC. In this study, we showed that AGR2 and AKR1B10 expressions in NPC tissues were associated with the prognosis of NPC patients, the patients with highly expressed AGR2 and AKR1B10 had a poor prognosis. Consistently, the serum levels of AGR2 and AKR1B10 were markedly higher in NPC patients compared with healthy control, and these concentrations decreased after treatment. Additionally, there was a positive linear correlation between AGR2 and AKR1B10 levels in NPC patients. Furthermore, AGR2 and AKR1B10 showed a positive relationship with Tumor Node Metastasis (TNM) grade and metastasis. Overall, there may be a tight relationship between AGR2 and AKR1B10 expression, and both of them can be used as serum biomarkers to achieve early diagnosis and provide effective therapeutic targets for NPC patients.
Keywords: AGR2; AKR1B10; Serum biomarker; Therapeutic target; Nasopharyngeal carcinoma
Abbreviations: NPC: Nasopharyngeal Carcinoma; AGR2: Anterior Gradient Protein 2; AKR1B10: Aldo- Keto Reductase 1B10; EBV: Epstein-Barr Virus; MMP: Matrix Metalloproteinase; UPR: Unfolded Protein Response; ER: Endoplasmic Reticulum; OS: Overall Survival; ECM: Extracellular Matrix; EGFR: Epidermal Growth Factor Receptor; IGF-1: Insulin-Like Growth Factor-1; DAG: Diacylglycerol; PKC: Protein Kinase C; CTL: Cytotoxic T Lymphocyte; PDI: Protein-Disulfide Isomerase
Introduction
Nasopharyngeal Carcinoma (NPC) is a commonly encountered type of malignant cancer,
which arises from epithelial cells of the nasopharynx and shows a remarkably high prevalence
in east Africa and Asia [1], especially South China, such as Guangdong, Guangxi and Hunan
[2,3]. Increasing evidence has suggested that the etiology of NPC is not only involved with
Epstein-Barr Virus (EBV) infection [4], chemical carcinogens and environmental exposures,
but also tightly correlated with genetic lesions [5,6]. Furthermore, the unusual incidence
patterns of NPC indicate that NPC high-risk genes play an aberrant role in Chinese and
Chinese-related populations, resulting in the inheritance and familial aggregation of NPC
[7]. The metastasis and invasion have been identified as significant symptoms of NPC, which
contributes to unfavorable curative effect and low survival rate [8]. To improve prognosis and
survival rate, novel therapeutic targets of NPC are urgently to be identified.
The previous studies have found that Anterior Gradient Protein 2 (AGR2) and Aldo-Keto
Reductase 1B10 (AKR1B10) are associated with NPC development and metastasis [9,10].
AGR2 is characterized as a member of the Protein-Disulfide Isomerase (PDI) family, with the
ability to modulate Unfolded Protein Response (UPR) proteins synthesis and Endoplasmic
Reticulum (ER) stress-associated pathways [11]. Cancer-secreted AGR2 is a potential
diagnostic and prognostic biomarker, it has a significant correlation with low Overall Survival
(OS) in multiple types of cancers, including prostate cancer [11,12], breast cancer [13], ovarian
cancer [14], lung adenocarcinoma cancer [15], Gastric Cancer (GC) [16], Colorectal Cancer (CRC) [17] and Non-Small Cell Lung Cancer (NSCLC) [18]. Aldo-keto
reductases (AKRs) are a group of monomeric cytoplasmic proteins,
including AKR1B1 and AKR1B10 [19]. AKR1B10, a detoxification
enzyme, is normally expressed in the gastrointestinal tract and
catalyses cofactor-dependent oxidation-reduction reactions [19]. Of
particular, AKR1B10 is secreted through lysosome-mediated nonclassical
pathway, then contributing to the increase of AKR1B10
in the serum. Functionally, AKR1B10 is positively associated with
tumor size and lymph node metastasis [20], further leading to
reduced survival in various malignancies, including Hepatocellular
Carcinoma (HCC) [21], Oral Squamous Cell Carcinoma (OSCC) [22],
lung adenocarcinoma [23] and GC [24]. Taken together, AGR2 and
AKR1B10 are highly expressed in multiple human solid cancers
and reported to promote the aggressive characteristics. Thus, they
might play an indispensable role in enhancing NPC malignancy. To
profoundly explore whether the serum levels of AGR2 and AKR1B10
can be considered as diagnostic biomarkers and therapeutic
targets of NPC, our study would detect the expression of AGR2
and AKR1B10 in tissue and serum samples from NPC patients
and control group. Further, we would combine with the analysis
of clinical data to assess the feasibility of AGR2 and AKR1B10 as
serum markers to achieve early diagnosis and provide effective
therapeutic targets for NPC patients.
Materials and Methods
Reagents and antibodies
Antibody against AGR2 was purchased from Bior Byt Company (San Francisco California, United States). Antibody against AKR1B10 was purchased from Abcam Company (Shanghai, China). Elisa Kit against AGR2 was purchased from Mlbio Company (Shanghai, China). Elisa Kit against AKR1B10 was purchased from Light of Life Company (Changsha, Hunan).
Human serum samples
106 NPC patients were enrolled in this study, and the patients were obtained from Hunan Cancer Hospital & the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University (Changsha, China) from July 2019 to March 2020. The patients were pathologically diagnosed as NPC. Of them, 50 pairs of NPC tissue samples and matched normal tissues were obtained by biopsy. Blood samples were obtained by venepuncture from 106 NPC patients, 106 corresponding NPC patients after treatment and 40 healthy donors. Then samples were clotted at 4-8 °C and centrifuged at 3000rpm for 10min. The collected serum was saved in 200μL EP tubes and stored at -80 °C until used. The 106 NPC patients contain 83 males and 23 females, their features were collected, including age, gender, EBVCA-IgG, EBVEA-IgA and TNM stages. The median age of the 106 patients was 48.5 years (range 36-78). The tumor histology and stages were classified according to the World Health Organization (WHO) classification and the TNM staging system of the Union for International Cancer Control (UICC), respectively. 40 healthy relatives of the NPC patients consisted of 22 males and 18 females. The median age of the 40 healthy relatives was 42 years (range 25-68), with no known diseases. All procedures were consistent with the National Institutes of Health Guide and approved by institutional board with patients’ written consent. This study was evaluated and approved by the Ethics Committee of the Affiliated Cancer Hospital of Xiangya Medical School, Central South University.
Immunohistochemistry staining and intensity analysis
Immunohistochemistry for protein expression was performed using SP Rabbit & Mouse HRP Kit (CW2069, CWBIO, Beijing, China) according to the manufacturer’s instructions. The paraffin sections were deparaffinized, rehydrated and washed in PBS. Antigen retrieval was performed in sodium citrate buffer in a microwave oven. After being blocked with 3% hydrogen peroxide, the tissue sections were permeabilized with 0.1% Triton X-100 and next immersed in 5% BSA. The primary antibodies (1:100) were added in an Immuno Histo Chemistry (IHC) Antibody Diluent, and incubation overnight. Subsequently, the sections were rinsed with PBS and incubated with a Horseradish Peroxidase (HRP)-conjugated Goat anti Rabbit IgG polyclonal antibody. After being rinsed with PBS, the section was incubated with SABC. The signal was developed with the peroxidase substrate DAB which appears as a brown reaction product or with AEC which appears as a red reaction product. All sections were counterstained with haematoxylin-eosin and were imaged under a microscope. The nucleus/cytoplasm was stained to yellow or brownish yellow (when the color was deep, it was brown), cells were considered to be positive for the target genes. Average IOD was the ratio of the accumulated optical density and the area of the sample under the visual field (for visual field of a 400X light microscope).
Haematoxylin-eosin staining
Tumor tissues were fixed with 4% paraformaldehyde for 24- 72h, then dehydrated and treated with xylene until becoming transparent, embedded by paraffin, and sliced in 8-um-thick sections by using a microtome. The sections were placed on gummed paper, dewaxed and stained with haematoxylin-eosin. The prepared sections were placed under the microscope to observe for pathological changes in tumor tissues.
Enzyme-linked immunosorbent assay
Enzyme-Linked Immunosorbent Assay (ELISA) was performed following the manufacture suggested protocol. First, standard and unknown concentration samples were added into the microplate. Then, the substance to be tested and the biotin labeled antibody were incubated at the same time. After washing, HRP labeled with avidin was added. After incubation and washing, the unbound enzyme conjugates were removed, and substrate A and B were added to act simultaneously with the enzyme conjugates. Finally, the color depth is proportional to the concentration of the substance to be measured in the sample.
Statistical analysis
SPSS 21.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA) were used for statistical analysis. The relationship of AGR2 with AKR1B10 concentrations was analyzed using Spearman. The differences between groups were statistically examined using Mann-Whitney U test. Using median values as the cut-off value for the marker, the receiver operating characteristic (ROC) analysis was done. Differences between groups were considered as *, P< 0.05; **, P< 0.01; or ***, P<0.001.
Results
Highly expressed AGR2 and AKR1B10 in human NPC biopsy tissues
AGR2 and AKR1B10 are overexpressed in multiple human cancers, and are associated with cancer metastasis, therapeutic resistance [25], pro-inflammatory phenotype acquisition [26], Extracellular Matrix (ECM) remodeling [27], angiogenesis generation [28], autophagy regulation [29], proliferation and apoptosis [30,31]. In order to verify the clinical significance of AGR2 and AKR1B10 in NPC patients, we collected 50 pairs of NPC tissue samples. IHC data showed that the positive expression rate of AGR2 and AKR1B10 in NPC tissues was remarkably higher than those in matched normal tissues (Figure 1A). Then, the IOD/ Area values of AGR2 and AKR1B10 were calculated, suggesting that there was a significant liner correlation between AGR2 and AKR1B10 expression in NPC patients (P<0.01, r=0.98) (Figure 1B). These results strongly demonstrated that highly expressed AGR2 and AKR1B10 might play a prominent role in accelerating NPC development.
Figure 1:The highly expressed AGR2 and AKR1B10 in human NPC biopsy tissues
A. Immunohistochemistry staining detected AGR2 and AKR1B10 expressions in primary NPC tissues and
matched normal tissues,
B. AGR2 and AKR1B10 expressions were quantitatively analyzed. The correlation between AGR2 and AKR1B10
IOD/Areas values was analyzed using spearman (P<0.01, r=0.98). These data were representative of 3 separate
experiments in triplicate. Data were presented as means ±S.D. of three independent experiments and were
statistically analyzed using Student’s t test. **, P< 0.01.

The concentrations of AGR2 and AKR1B10 in NPC patients
AGR2 and AKR1B10 oncoproteins are secreted by cancer cells, and the serum levels of AGR2 and AKR1B10 are not only used to predict cancer progression and prognosis, but also evaluate therapeutic efficiency [32]. To evaluate the values of AGR2 and AKR1B10 in NPC patient treatment, we collected serum samples from NPC patients at pre-therapy and post-therapy, and healthy population served as control. ELISA assay was performed to detect AGR2 and AKR1B10 concentrations in the serum samples. The results showed that the median concentration of AGR2 is 1.46ng/ mL in the NPC group, 0.78ng/mL in the NPC after treatment group and 0.55ng/mL in the healthy control. The median concentration of AKR1B10 is 156.87pg/mL in the pre-therapy NPC group, 118.65pg/mL in the post-therapy group and 77.37pg/mL in the healthy control (Table 1). These indicated that serum AGR2 and AKR1B10 concentrations were higher in NPC patients than those in the healthy control (P< 0.01; P<0.001). Meanwhile, the AGR2 and AKR1B10 concentrations significant decreased after treatment (P< 0.05; P< 0.01). However, there was no significant difference between post-therapy NPC and healthy control (Figure 2A & 2B).
Figure 2:The concentrations of AGR2 and AKR1B10 in the serum samples from the NPC patients at pretreatment
and post-treatment
A. ELISA assay was used to detect serum AGR2 in NPC patients at pre-treatment and post-treatment and
healthy population,
B. AKR1B10 was detected in the serum samples from at pre-treatment and post-treatment and healthy
population. These data were representative of 3 separate experiments in triplicate. Data were presented as
means ±S.D. of three independent experiments and were statistically analyzed using Student’s t test. *, P<0.05;
**, P< 0.01; or ***, P<0.001.
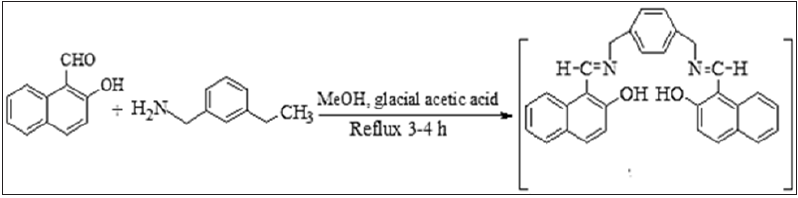
Table 1: Serum concentrations of AGR2 and AKR1B10 in NPC patients.
The association of AGR2 and AKR1B10 with clinical characteristics
The association of AGR2 and AKR1B10 levels with and NPC patients’ clinical characteristics was further analyzed. The clinicopathological traits of NPC patients were summarized in (Table 2), containing age, gender, EBVCA-IgG, EBVEA-IgA, and TNM stages. The results showed that AGR2 and AKR1B10 serum levels displayed a remarkable correlation with TNM grade (P<0.05) and metastasis (P<0.05). But there were no significant relationships of AGR2 and AKR1B10 concentrations with age, gender, EBVCA-IgG or EBVEA-IgA (Table 2). These data strongly suggested that highly expressed AGR2 and AKR1B10 may be used markers to predict NPC metastasis.
Table 2: The relevance of AGR2 and AKR1B10 levels with clinical features.
Combination of AGR2 with AKR1B10 as potential therapeutic for NPC patients
The above results showed that AGR2 and AKR1B10 concentrations significantly increased in pre-therapy NPC patients, while all of them decreased in post-therapy patients at the same time points. To further explore whether there was a linear correlation between AGR2 and AKR1B10 concentrations, we analyzed the levels of AGR2 and AKR1B10 in the same NPC patients. Data showed that there was a positive linear correlation between AGR2 and AKR1B10 concentrations in NPC patients before treatment (P<0.01, r=1.00) (Figure 3). However, there was no significance in the corresponding NPC patients after treatment. Next, we further want to determine whether the clinical performance of AGR2 and AKR1B10 could be served as evaluation criteria of therapeutic effect for NPC patients by performing ROC statistics. Based on the AGR2 and AKR1B10 protein levels in serum samples, we found that AGR2 cut-off value of 0.83ng/mL had a sensitivity of 48.1% and a specificity of 90%, and AGR2 median value of 0.77ng/mL had a sensitivity of 50% and a specificity of 70% (Figure 4A). AKR1B10 cut-off value of 93.57pg/mL had a sensitivity of 75.5% and a specificity of 70%, and AKR1B10 median value of 140.45pg/mL had a sensitivity of 50% and a specificity of 90% (Figure 4B). Meanwhile, combined ROC curve analysis for AGR2 and AKR1B10 was used to verify the clinical value of AGR2 and AKR1B10 (Figure 4C).
Figure 3:The relativity of AGR2 with AKR1B10 expressions in serum samples from NPC patients. AGR2 and AKR1B10 concentrations were detected in serum samples from NPC patients suing ELISA assay. The relationship of AGR2 with AKR1B10 concentrations was analyzed using Spearman (P<0.01, r=1.00). **, P< 0.01.
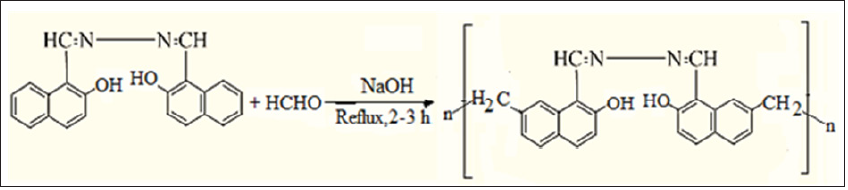
Figure 4: Combination of AGR2 and AKR1B10 as potential blood-based diagnostic and therapeutic markers for
NPC patients
A. ROC curve analysis of AGR2 for NPC diagnostic and therapeutic marker
B. ROC curve analysis of AGR2 for NPC diagnostic and therapeutic marker
C. Combined ROC curve of AGR2 and AKR1B10 for NPC diagnostic and therapeutic markers. **, P<0.01; ***,
P<0.001. ROC: Receiver Operating Characteristic, AUC: Area Under the Curve, 95% CI: 95% Confidence Interval.
Discussion
In clinical, most of confirmed NPC patients are in advanced
stage due to the delayed diagnosis, they are often characterized by
distant metastasis, poor prognosis and low OS rate [33]. Distant
metastasis is still the major reason for NPC treatment failure
[34], and early discovery, early intervention and treatment are
essential for improving the therapeutic efficiency for NPC patients.
Currently, increasing number of early detection methods are
emerging, and our research also aims to look for sensitive and
accurate biomarkers for early diagnosis. AGR2 and AKR1B10 have
been identified as novel serum protein biomarkers to monitor
progression due to their potential roles in promoting invasion
and metastasis of numerous malignant cancers [20,35,36]. Of
note, AGR2 and AKR1B10 proteins are significantly higher in NPC
tissues compared with the matched normal nasopharyngeal tissues
[22,37]. Consistently, some clinical studies have also found the
increased AGR2 and AKR1B10 concentrations in serum or plasma
samples of NPC patients compared with healthy control, implying
the diagnostic significance of AGR2 and AKR1B10 for NPC [37]. In
this study, we demonstrated that AGR2 and AKR1B10 expressions
were remarkably up regulated in human primary NPC tissues
compared to the matched normal nasopharyngeal tissues, and
there was a positive liner correlation between AGR2 and AKR1B10
IOD/Areas values. Thus, there may be a tight correlation between
AGR2 and AKR1B10 expression, and both of them can cooperatively
serve as diagnostic biomarkers for NPC.
Growing number of experimental studies have indicated that
silencing of AKR1B10 would restrain cancer metastasis and induce
cancer cells apoptosis [19]. And the inhibitors of AKR1B10 can
be utilized as adjuvant drugs to overcome chemoresistance in
numerous cancers [38]. For instance, AKR1B10 inhibitor epalrestat
facilitates sorafenib (an anti-cancer drug)-induced apoptosis and
autophagy through inhibiting the activation of mTOR pathway in
HCC [39]. In agreement with this, silencing AGR2 can inhibit cell
proliferation, reduce invasion and dissemination, induce cancer
cells death and improve anti-cancer therapy [30,40,41]. Moreover,
the previous studies have found that AGR2 can serve as a cancerassociated
antigen for facilitating anti-cancer immunotherapy
through triggering AGR2 peptide-specific Cytotoxic T Lymphocyte
(CTL) response and providing a potent candidate for antibody
targeting, which offer a favorable avenue to improve NPC
therapeutic efficiency [42,43]. Based on these works, we speculated
that serum AGR2 and AKR1B10 may be potential markers for NPC
therapy. To validate the above speculation, we detected AGR2 and
AKR1B10 in the serum samples from the patients at pre-therapy
and post-therapy. The results showed that AGR2 and AKR1B10
concentrations were increased in the NPC patients when compared
with the healthy group, while they decreased in the post-treatment
patients. There also was a positive relationship between AGR2
and AKR1B10 levels in NPC patients before treatment. Further,
the clinical analysis showed that serum AGR2 and AKR1B10 were
closely related to the TNM stages and metastasis of NPC patients.
ROC curve analysis was used to evaluate the clinical performance of
AGR2 and AKR1B10 as therapeutic markers for NPC patients. AGR2
and AKR1B10 could be considered as collaborative serum markers
for NPC patients. Collectively, AGR2 and AKR1B10 might not
only be potential diagnostic and prognostic biomarkers, but also
characterized as promising therapeutic targets for NPC patients.
Besides, it is worth noting that there may is a closely relationship
between AGR2 and AKR1B10 expression, but the regulatory
mechanisms remain to be further investigated.
Author contributions
Yuan Tan drafted the manuscript. Renhua Fan, Liguang Lin, Fang Liu and Meihua Zhou carried out the assays and collected the samples. Yuan Tan and Lei Wang conducted the study design. Lei Wang revised the manuscript. All authors reviewed and approved the final manuscript.
References
- Yang M, Huang W (2020) Circular RNAs in nasopharyngeal carcinoma. Clin Chim Acta 508: 240-248.
- Kontny U, Franzen S, Behrends U, Buhrlen M, Christiansen H, et al. (2016) Diagnosis and treatment of nasopharyngeal carcinoma in children and adolescents-recommendations of the GPOH-NPC study group. Klin Padiatr 228(3): 105-112.
- Feng BJ, Huang W, Shugart YY, Lee MK, Zhang F, et al. (2002) Genome-wide scan for familial nasopharyngeal carcinoma reveals evidence of linkage to chromosome 4. Nat Genet 31(4): 395-399.
- Verma N, Patel S, Osborn V, McBride S, Riaz N, et al. (2020) Prognostic significance of human papillomavirus and Epstein-bar virus in nasopharyngeal carcinoma. Head Neck 42(9): 2364-2374.
- Bei JX, Zuo XY, Liu WS, Guo YM, Zeng YX (2016) Genetic susceptibility to the endemic form of NPC. Chin Clin Oncol 5(2): 1-15.
- Imamura M, Kawai T, Okada S, Izawa K, Takachi T, et al. (2011) Disseminated BCG infection mimicking metastatic nasopharyngeal carcinoma in an immunodeficient child with a novel hypomorphic NEMO mutation. J Clin Immunol 31(5): 802-810.
- Simons MJ (2011) Nasopharyngeal carcinoma as a paradigm of cancer genetics. Chin J Cancer 30(2): 79-84.
- Xu T, Su B, Huang P, Wei W, Deng Y, et al. (2017) Novel biomarkers of nasopharyngeal carcinoma metastasis risk identified by reverse phase protein array based tumor profiling with consideration of plasma Epstein-Barr virus DNA load. Proteomics Clin Appl 11(5-6).
- Li Y, Lu J, Peng Z, Tan G, Liu N, et al. (2014) N,N'-dinitrosopiperazine-mediated AGR2 is involved in metastasis of nasopharyngeal carcinoma. PLoS One 9(4): e92081.
- He YC, Shen Y, Cao Y, Tang FQ, Tian DF, et al. (2016) Overexpression of AKR1B10 in nasopharyngeal carcinoma as a potential biomarker. Cancer Biomark 16(1): 127-135.
- Jia M, Guo Y, Zhu D, Zhang N, Li L, et al. (2018) Pro-metastatic activity of AGR2 interrupts angiogenesis target bevacizumab efficiency via direct interaction with VEGFA and activation of NF-κB pathway. Biochim Biophys Acta Mol Basis Dis 1864(5): 1622-1633.
- Vitello EA, Quek SI, Kincaid H, Fuchs T, Crichton DJ, et al. (2016) Cancer-secreted AGR2 induces programmed cell death in normal cells. Oncotarget 7(31): 49425-49434.
- Guo J, Gong G, Zhang B (2017) Identification and prognostic value of anterior gradient protein 2 expression in breast cancer based on tissue microarray. Tumour Biol 39(7).
- Sung HY, Choi EN, Lyu D, Park AK, Ju W, et al. (2014) Aberrant hypomethylation-mediated AGR2 overexpression induces an aggressive phenotype in ovarian cancer cells. Oncol Rep 32(2): 815-820.
- Milewski D, Balli D, Ustiyan V, Le T, Dienemann H, et al. (2017) FOXM1 activates AGR2 and causes progression of lung adenomas into invasive mucinous adenocarcinomas. PLoS Genet 13(12).
- Zhang J, Jin Y, Xu S, Zheng J, Zhang QI, et al. (2016) AGR2 is associated with gastric cancer progression and poor survival. Oncol Lett 11(3): 2075-2083.
- Tian S, Hu J, Tao K, Wang J, Chu Y, et al. (2018) Secreted AGR2 promotes invasion of colorectal cancer cells via Wnt11-mediated non-canonical Wnt signaling. Exp Cell Res 364(2): 198-207.
- Alavi M, Mah V, Maresh EL, Bagryanova L, Horvath S, et al. (2015) High expression of AGR2 in lung cancer is predictive of poor survival. BMC Cancer 15: 655.
- Huang L, He R, Luo W, Zhu YS, Li J, et al. (2016) Aldo-keto reductase family 1 member B10 inhibitors: Potential drugs for cancer treatment. Recent Pat Anticancer Drug Discov 11(2): 184-196.
- Ma J, Luo DX, Huang C, Shen Y, Bu Y, et al. (2012) AKR1B10 overexpression in breast cancer: Association with tumor size, lymph node metastasis and patient survival and its potential as a novel serum marker. Int J Cancer 131(6): E862-E871.
- Shi J, Chen L, Chen Y, Lu Y, Chen X, et al. (2019) Aldo-keto reductase family 1 member B10 (AKR1B10) overexpression in tumors predicts worse overall survival in hepatocellular carcinoma. J Cancer 10(20): 4892-4901.
- Fang CY, Lin YH, Chen CL (2019) Overexpression of AKR1B10 predicts tumor recurrence and short survival in oral squamous cell carcinoma patients. J Oral Pathol Med 48(8): 712-719.
- Hung JJ, Yeh YC, Hsu WH (2018) Prognostic significance of AKR1B10 in patients with resected lung adenocarcinoma. Thorac Cancer 9(11): 1492-1499.
- Ahmed SMU, Jiang ZN, Zheng ZH, Li Y, Wang XJ, et al. (2019) AKR1B10 expression predicts response of gastric cancer to neoadjuvant chemotherapy. Oncol Lett 17(1): 773-780.
- Li J, Hu J, Luo Z, Zhou C, Huang L, et al. (2019) AGR2 is controlled by DNMT3a-centered signaling module and mediates tumor resistance to 5-Aza in colorectal cancer. Exp Cell Res 385(1): 111644.
- Dumartin L, Alrawashdeh W, Trabulo SM, Radon TP, Steiger K, et al. (2017) ER stress protein AGR2 precedes and is involved in the regulation of pancreatic cancer initiation. Oncogene 36(22): 3094-3103.
- Fessart D, Domblides C, Avril T, Eriksson LA, Begueret H, et al. (2016) Secretion of protein disulphide isomerase AGR2 confers tumorigenic properties. Elife 5.
- Pan F, Yang W, Li W, Yang XY, Liu S, et al. (2017) Conjugation of gold nanoparticles and recombinant human endostatin modulates vascular normalization via interruption of anterior gradient 2-mediated angiogenesis. Tumour Biol 39(7).
- Worfolk JC, Bell S, Simpson LD, Carne NA, Francis SL, et al. (2019) Elucidation of the AGR2 interactome in esophageal adenocarcinoma cells identifies a redox-sensitive chaperone hub for the quality control of MUC-5AC. Antioxid Redox Signal 31(15): 1117-1132.
- Alsereihi R, Schulten HJ, Bakhashab S, Saini K, Al Hejin AM, et al. (2019) Leveraging the role of the metastatic associated protein anterior gradient homologue 2 in unfolded protein degradation: A novel therapeutic biomarker for cancer. Cancers (Basel) 11(7): 890.
- Li J, Guo Y, Duan L, Hu X, Zhang X, et al. (2017) AKR1B10 promotes breast cancer cell migration and invasion via activation of ERK signaling. Oncotarget 8(20): 33694-33703.
- Sommerova L, Ondrouskova E, Vojtesek B, Hrstka R (2017) Suppression of AGR2 in a TGF-beta-induced smad regulatory pathway mediates epithelial-mesenchymal transition. BMC Cancer 17(1): 546.
- Sun XS, Liang YJ, Liu SL, Chen QY, Guo SS, et al. (2019) Subdivision of nasopharyngeal carcinoma patients with bone-only metastasis at diagnosis for prediction of survival and treatment guidance. Cancer Res Treat 51(4): 1259-1268.
- Li W, Bai Y, Wu M, Shen L, Shi F, et al. (2017) Combined CT-guided radiofrequency ablation with systemic chemotherapy improves the survival for nasopharyngeal carcinoma with oligometastasis in liver: Propensity score matching analysis. Oncotarget 8(32): 52132-52141.
- Curtarelli RB, Goncalves JM, Dos Santos LGP, Savi MG, Nor JE, et al. (2018) Expression of cancer stem cell biomarkers in human head and neck carcinomas: A systematic review. Stem Cell Rev Rep 14(6): 769-784.
- Pan F, Li W, Yang W, Yang XY, Liu S, et al. (2018) Anterior gradient 2 as a supervisory marker for tumor vessel normalization induced by anti-angiogenic treatment. Oncol Lett 16(3): 3083-3091.
- Li Y, Wang W, Liu Z, Jiang Y, Lu J, et al. (2018) AGR2 diagnostic value in nasopharyngeal carcinoma prognosis. Clin Chim Acta 484: 323-327.
- DiStefano JK, Davis B (2019) Diagnostic and prognostic potential of AKR1B10 in human hepatocellular carcinoma. Cancers (Basel) 11(4): 486.
- Geng N, Jin Y, Li Y, Zhu S, Bai H (2020) AKR1B10 inhibitor epalrestat facilitates sorafenib-induced apoptosis and autophagy via targeting the mTOR pathway in hepatocellular carcinoma. Int J Med Sci 17(9): 1246-1256.
- Liu R, Qian M, Zhou T, Cui P (2019) TP53 mediated miR-3647-5p prevents progression of cervical carcinoma by targeting AGR2. Cancer Med 8(13): 6095-6105.
- Negi H, Merugu SB, Mangukiya HB, Li Z, Zhou B, et al. (2019) Anterior gradient-2 monoclonal antibody inhibits lung cancer growth and metastasis by upregulating p53 pathway and without exerting any toxicological effects: A preclinical study. Cancer Lett 449: 125-134.
- Lee HJ, Hong CY, Jin CJ, Kim MH, Lee YK, et al. (2012) Identification of novel HLA-A*0201-restricted epitopes from anterior gradient-2 as a tumor-associated antigen against colorectal cancer. Cell Mol Immunol 9(2): 175-183.
- Liu AY, Kanan AD, Radon TP, Shah S, Weeks ME, et al. (2019) AGR2, a unique tumor-associated antigen, is a promising candidate for antibody targeting. Oncotarget 10(42): 4276-4289.
© 2021 © Lei Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






