- Submissions

Full Text
Significances of Bioengineering & Biosciences
Selected Sudanese Medicinal Plants Induce Anticancer and Cytotoxic Effects in Cervical Cancer Cell Line
Montasir Ahmed Elnour1*, Asaad Khalid1 and Fatima Penech2
1 National Center for Research, Sudan
2 International Center for Chemical and Biological Sciences, Pakistan
*Corresponding author:Montasir Ahmed Elnour, Medicinal and Aromatic Plants Research Institute, National Center for Research, Post Box Number: 2404, Khartoum, Sudan
Submission: June 11, 2018;Published: July 19, 2018

ISSN 2637-8078Volume2 Issue3
Abstract
Modern pharmacology, however, relies on refined chemicals-either obtained from plants, or synthesized. This work investigated the anticancer, antioxidant and Cyto toxicity activities of N. lotus leaves citrate, N. lotus leaves not citrate, P. juliflora leaves commonly used as anti-inflammatory and Ant diabetic. All the plant parts were extracted using 80% methanol, the anticancer activity was examined by using MTT assay against Hela (Cervical Cancer) Cell Line. And determine their antioxidant activities by testing DPPH cytotoxicity using-(4, 5-Dimethyl thiazole-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT), filter and kept in dark, prepared freshly. The examined plants methanolic extracts of P. juliflora leaves is high activity against Hela (Cervical Cancer) Cell Line IC50 is 56.02μg/ml. The extract C N. lotus leaves citrate, N. lotus leaves not citrate has shown none active anti- Hela (Cervical Cancer) IC50 values 100, and >100μg/ml respectively. All the extracts revealed cytotoxicity activity not toxic in N. lotus leaves citrate, N. lotus leaves not citrate, P. juliflora leaves the inhibition percentage with (90.81,89.33074,86.47866) (73.17427,71.93975,60.17069) (74.93001,73.78714, 71.13981) respectively. The following plant parts showed highly potent scavenging activity against DPPH (above 80%); N. lotus leaves citrate, 88.78, While the following revealed a good activity against DPPH (between 60-79); N. lotus leaves not citrate, P. juliflora leaves with inhibition% are 77and 62.96 respectively.
Keywords: Anticancer; Medicinal plants; Cytotoxicity; Cervical cancer
Introduction
Cancer harms the body when damaged cells divide uncontrollably to form lumps or masses of tissue called tumors (except in the case of leukemia where cancer prohibits normal blood function by abnormal cell division in the blood stream). Tumors can grow and interfere with the digestive, nervous, and circulatory systems and they can release hormones that alter body function. Tumors that stay in one spot and demonstrate limited growth are generally considered to be benign.
The tradition for developing plant-based drugs in modern Western medicine is largely based on a paradigm (model) that there is a single active ingredient in medicinal plants, or at least a primary chemical, that is responsible for the medical effectiveness. However, it may be that many preparations used in traditional herbal medicine are effective because of synergistic (interactive) therapeutic effects of several ingredients. Certainly many traditional herbal drug preparations are compounded from several plants. Such drug mixtures are not of interest to pharmaceutical firms, because they generally cannot be patented (although under some conditions natural products can secure patent protection). On the other hand, as a visit to a pharmacy or “health-food” store quickly reveals, numerous companies are marketing plant mixtures as “dietary supplements,” which are in fact being utilized as nonprescription drugs, although there is generally limited or no modern research proof of effectiveness. Since the private sector has limited interest in this issue, there is a clear need for public supported (government) research Balandrin et al. [1].
Estimates for the number of described species of flowering plants in the world vary from 223 300 [2] to about 315 903 [3]. In the US, almost 1800 medicinal plant species are commercially available Muller & Clauson [4]. It has been estimated that about 13,000 species of plants have been employed for at least a century as traditional medicines by various cultures around the world [5]. Very likely a much larger number of the world’s flowering plant species have been used medicinally. Sometimes the figure of 70,000 medicinal plant species is cited, but this includes many algae, fungi, and micro-organisms that are not really plants as the word is understood by botanists. In any event, there is no other category of plants useful to man (with the possible exception of ornamental plants) that includes so many species, and the question naturally arises why such a staggering number of plants have useful medicinal properties Tyler [5].
Material and Methods
Collection of tested plant parts of the N. lotus leaves citrate, N. lotus leaves not citrate, P. juliflora leaves Collected from the Farm of Medicinal and Aromatic Plants Research Institute, Khartoum, Sudan (MAPRI) and identified of taxonomist team of Medicinal and Aromatic Plants Research Institute, National Center of Research, Khartoum, Sudan. And herbarium voucher was deposited at herbarium medicinal plants in the MAPRI.
Preparation of crude plant extract
One hundred grams of each plant sample was art coarsely powdered using Mortar and pistil and extracted with 80% methanol for 18 hours using shaker (Stuart scientific, flash shaker, SF 1, UK). The extract was filtered and evaporated using rotary evaporator at 40 °C (Buchi, 461, Switzerland).
Culture media and human tumor cell lines
PC3 (prostate cancer cell line) were obtained frozen in liquid nitrogen (-180 °C), the tumor cell lines were maintained in the Institute of ICCB, University of Karachi Pakistan.
Culture media
RPMI-1640 medium was used for culturing and maintenance of the human tumor cell lines. The medium was supplied in a soluble form. Before using the medium it was warm at 37 °C in a water bath and supplemented with penicillin/streptomycin and Fetal bovine serum (FBS) with 10% concentration. The cells were maintained at 37 °C in a humidified atmosphere with 5% CO2 and were sub cultured twice a week.
Procedure
Maintenance of the human cancer cell lines in the laboratory
A cryo tube containing frozen cells was taken out of the liquid nitrogen container and then thawed in a water bath at 37 °C. The cryo tube was opened under strict aseptic conditions and its content was supplied by 5ml complete media (RPMI-1640 with 10% fetal bovine serum) drop by drop in a 50ml disposable sterile falcon tubes and were centrifuged at 1200rpm for 10min to discard the preserving solution. The supernatant was discarded, and the cell pellet was seeded in 5ml complete media in T25 Nunclon sterile tissue culture flasks. The cell suspension was incubated at 37 °C in a humidified atmosphere with 5% CO2 and followed up daily with changing the supplemented medium every 2-3 days. Incubation was continued until a confluent growth was achieved and the cells were freshly sub cultured before each experiment.
Collection of cells by trypsinization
The media was discarded. The cell monolayer was washed twice with 5ml phosphate buffered saline and all the adherent cells were dispersed from their monolayer by the addition of 1ml trypsin solution (0.025% trypsin w/v) for 2 minutes. The flask was left in the incubator till complete detachment of all the cells and checked with the inverted microscope (Olympus). Trypsin was inactivated by the addition of 5ml of the complete media. The trypsin content was discarded by centrifugation at 1200rpm for 10 minutes. The supernatant was discarded, and the cells were separated into single cell suspension by gentle dispersion several times, then suspended and seeded in 5 ml complete media in T25 Nunclon sterile tissue culture flasks.
Determination and counting of viable cells
50μl of fresh culture media was added to 50μl of the single cell suspension. The cells were examined under the inverted microscope using the hemo cytometer. Viable cells were counted, and the following equation was used to calculate the cell count /ml of cell suspension.
Figure 1:
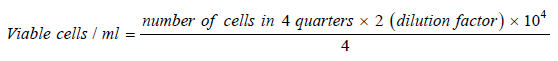
The cells were then diluted to give the concentration of single cell suspension required for each experiment. The cell count was adjusted to 1x104 -105 cells/ml using medium containing 10% fetal bovine serum.
Cryopreservation of cells
To avoid the loss of the cell line, excess cells were preserved in liquid nitrogen as follows: Equal parts of the cell suspension and freezing medium (10% DMSO in complete media) were dispersed to cryo tubes. The cryo tubes were racked in appropriately labeled polystyrene boxes gradually cooled till reaching -80 °C. Then the cry tubes were transferred to a liquid nitrogen (-196 °C).
Microculture Tetrazolium (MTT) Assay
MTT assay
In order to evaluate the cytotoxicity effect of the extracts and compounds, the following procedure of the MTT was used.
MTT procedure
Serial dilutions of extract were prepared in a 96 well flat bottomed plate (Nalge Nunc, Inter.). The outer walls of the plate were filled with 250μl of in-complete culture medium except the last row 6 middle wells (B-G), which were used for the negative control receiving 50μl of culture medium and 2μl of sterile 0.5% Triton x.
To the rest of the plate, 50μl/wells (CCM) were added and 30 μl more were added to second column wells (B-G) that were used as first extract dilution wells. To the first dilution wells in the row, 500μg of c suspension extract were added to the 80μl. extract were then serially diluted by two-fold dilution from well B3 till B11 by transferring 250μl to the next well after proper mixing. From the last dilution wells (B-11), 50μl were discarded. Each compound was tested in triplicate. Cell suspension in a complete culture medium containing 2.5X105/ml was properly mixed, and 150μl of it were transferred into each well of the plate. The plate was covered and placed in 5% CO2 incubator at 37 °C for three-five days (72 hours-120 hours).
On the third/fifth day, the supernatant was removed from each well without detaching cells. MTT stock (5mg/ml) was prepared earlier in 100ml PBS. MTT suspension was vortexes and kept on magnetic stirrer until all MTT dissolved. The clear suspension was filtered sterilized with 0.2μ Millipore filter and stored at 4 °C or –20 until use. MTT was diluted (1:3.5) in a culture medium and brought to room temperature. To each well of the 96 well plates, 50μl of diluted MTT were added. The plate was incubated further at 37 °C for 2 to 3 hours in CO2 incubator. MTT was removed carefully without detaching cells, and 200μl of DMSO were added to each well. The plate was agitated at room temperature for 15minutes then read at 540nm using micro plate reader.
%Inhibition=[(A Control–A Sample)/A Control]×100
Where A Control is the absorbance of the negative control and A Sample the absorbance of tested samples or standard. All data are an average of triplicate analyses.
Antioxidant Assay
DPPH free radical scavenging activity method
A rapid, simple and inexpensive method to measure antioxidant capacity of food involves the use of the free radical, 2, 2-Diphenyl- 1-picrylhydrazyl (DPPH). DPPH is widely used to test the ability of compounds to act as free radical scavengers or hydrogen donors, and to evaluate antioxidant activity of foods. It has also been used to quantify antioxidants in complex biological systems in recent years. The odd electron in the DPPH free radical gives a strong absorption maximum at 517nm and is purple in color. The color turns from purple to yellow as the molar absorptive of the DPPH radical at 517nm when the odd electron of DPPH radical becomes paired with hydrogen from a free radical scavenging antioxidant to form the reduced DPPH-H. The resulting decolorization is stoichiometric with respect to number of electrons captured.
Procedure
The DPPH radical scavenging was determined according to the method of Shimada et al., (1992), with whose modification. In 96-wells plate, the test samples were allowed to react with 2.2Di (4-tert-octylphenyl)-1-picryl-hydrazyl stable free radical (DPPH) for half an hour at 37 °C. The concentration of DPPH was kept as (300μM). The test samples were dissolved in DMSO while DPPH was prepared in ethanol. After incubation, decrease in absorbance was measured at 517nm using multiplate reader spectrophotometer. Percentage radical scavenging activity by samples was determined in comparison with a DMSO treated control group. All tests and analysis were run in triplicate.
Percent of DPPH inhibition=[(AB-AA)/AB]×100
Where; AA and AB are the absorbance values of the test and of the blank sample, respectively. A percent inhibition versus concentration curve was plotted.
Statistical Analysis
All data are presented as mean ±standard deviation of the mean–statistical analysis for all the assays result were done using students t-test significance was tribute to probability values P< 0.05 or P< 0.01 in some cases .
Result and Discussion
Modern pharmacology, however, relies on refined chemicals - either obtained from plants, or synthesized. The first pure medicinal substance derived from plants was morphine, extracted from the opium poppy at the turn of the 19thcentury.Often; chemicals extracted from plants are altered to produce drugs. For example, dysgenic is obtained from various yam (Dioscorea) species of South America, and is converted to progesterone, the basis of the oral contraceptive pill. Aspirin-like chemicals were once obtained from willows (Salix species) and European meadowsweet (Filipendula ulmaria), but aspirin is now synthesized in the laboratory. Numerous medicines in use today are extracted from plants. About 50 to 60% of pharmaceutical drugs are either of natural origin or obtained through use of natural products as starting points in their synthesis Verlet [6]; Balandrin et al. [1]. Higher plants have given rise to about 120 commercial drugs and 10-25% of all prescription drugs contain at least one active compound from a higher plant Duke[6]; Cox & Balick [7].
P. juliflora leaves showed high activity against HeLa cell Lines (IC50 56.02μg/ml), as well as very high DPPH (IC50 40.81μg/ml). Leaves were found to be not toxic on normal cell lines (IC50 >100μg/ ml). The previous study showed that Prosopis juliflora contain many secondary metabolites compounds for example the leaves contain tannins, acids, glycosides, flavonoids and alkaloids, (Sathiya and Muthuchelian, 2008). The leaves can be used for forage. Providing good bee pasturage also, nectar from mesquite yields a superior honey. Bark, rich in tannin, is used for roofing in Colombia. The gum forms adhesive mucilage, used as an emulsifying agent. Gum is used in confectionary and mending pottery. Roots contain 6–7% tannin, which might discourage Rhizobia.
The juice is used in folk remedies for cancerous condition and reported to be cathartic, cyanogenetic, discutient, emetic, stomachic, and vulnerary, mesquite is a folk remedy for catarrh, colds, diarrhea, dysentery, excrescences, eyes, flu, headcold, hoarseness, inflammation, itch, measles, pinkeye, stomachache, sore throat, and wounds, Pima Indians drank the hot tea for sore throat Lewis and Elvin-Lewis [8]. Per 100g, the flowers is reported to contain (ZMB): 21.0g protein, 3.2g fat, 65.8g total carbohydrate, 15.5g fiber, 10.0g ash, 1,310mg Ca, and 400mg P. Leaves contain 19.0g protein, 2.9g fat, 69.6g total carbohydrate, total carbohydrate, 21.6g fiber, 8.5g ash, 2,080mg Ca, and 220g P. Fruits contain 13.9g protein, 3.0g fat, 78.3g total carbohydrate, 27.7g fiber, and 4.8g ash. Seeds contain (ZMB) 65.2g protein, 7.8g fat, 21.8g total carbohydrate, 2.8g fiber, and 5.2 g ash (Table 1).
Table 1: Yield percentage of the extracted with 80%methanol plants.
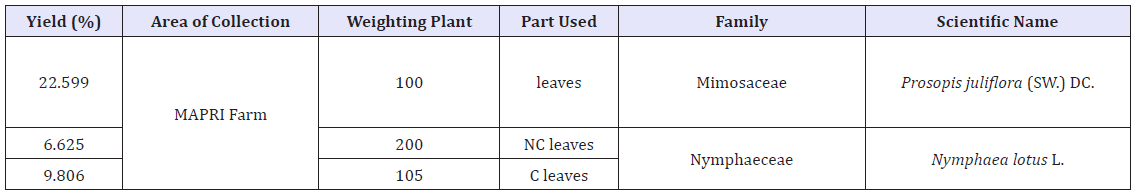
Note: C leaves means Nympheae lotus citrate leaves and NC leaves means Nympheae lotus not citrate leaves.
Another analysis of the fruit shows 14.35% water (hygroscopic), 1.64% oil, 16.36% starch, 30.25% glucose, 0.85% nitrogenous material, 5.81% tannin-like material, 3.5% mineral salts, and 27.24% cellulose (Table 2). Mesquite gum readily hydrolyses with dilute sulfuric acid to yield L-arabinose and D-galactose and 4-o-methyl-D-glucuronic acid at 4:2:1. Owing to the high content of arabinose, the gum is an excellent source of sugar. Roots contain 6.7% tannin, bark 3–8.4%, and dry wood 0.9%. The alkaloids 5-hydroxytryptamine and tryptamine are reported from this species (Simpson, 1977). Mesquite feeding to pigs was promising during the first four weeks, deteriorating thereafter, perhaps due to phytohemagglutinins and trypsin inhibition (Table 3). Feeding trials with sheep show a 15% higher protein digestibility coefficient for mesquite pods than for alfalfa hay Del Valle et al. [9]. Contains isorhamnetin 11 3-glucoside, apigenin 6, 8-diglycoside, and traces of quercitin 3’, 3diOMe, leutolin 3’-OMe, and apigenin diglycoside (Simpson, 1977) [10].
Table 2: IC50 of the methanol extracts of the selected Sudanese medicinal plants for cytotoxicity against Hela (cervical cancer) cell line proliferation.

Table 3: MTT cytotoxicity of 80% methanolic extract of selected Sudanese medicinal plants against Vero cell line..

Acknowledgement
Our gratefullness to Dr. Waeil Elsadig taxonomist team leader at MAPRI for the identification of plant species. Sincere thanks for Professor Aisha Zuheir Almagboul (MAPRI).
References
- Balandrin M, Kinghorn A, Farnsworth W (1993) Plant-derived natural products drug development. In: Kinghorn AD, Balandrin MF (Eds.), Human medicinal agents. American Chemical Society, Washington DC, USA, pp. 2-12.
- Scotland R, Wortley A (2003) How many species of seed plant are there? Taxon 52: 101 -104.
- Kier G, Peter R, Crane (2009) University of Chicago. Chicago, USA, Vol. 106(23).
- Muller J, Clauson K (1997) Pharmaceutical considerations of common herbal medicine. Am. J. Manage Care 3: 1753-1770.
- Tyler V (1993) The honest herbal. Pharmaceutical Products Press (Haworth Press) Binghamton, (3rd edn), New York, USA, p. 375.
- Verlet N (1990) New markets for herbs in France and Europe. The Herb Spices and Med Plant Digest 8(2): 1-5.
- Duke J (1993) Medicinal plants and the pharmaceutical industry. In: Janick J, Simon JE (Eds.), New Crops. John Wiley and Sons Inc, New York, USA, pp. 664-669.
- Lewis W, Elvin LM (1978) Medical botany, plants affecting manâ health. John Wiley & Sons Inc, New York, USA, Vol. 10(1).
- Del Valle FG (1983) El Tiwanaku Clásico en Azapa. Documentos de Trabajo 3: 94-113.
- Cox P, Balick M (1994) The ethnobotanical approach to drug discovery. Sci Am 270(6): 82-87.
© 2018 Montasir Ahmed Elnour. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






