- Submissions

Full Text
Research in Pediatrics & Neonatology
Escherichia Coli Resistance to Amoxicillin-Clavulanic Acid in Pediatric Urinary Tract Infections
Sandra Hilbert1, Mana Hedjri2, Michael Surböck1 and Milen Minkov1,2,3*
1Floridsdorf Hospital, Department of Pediatrics & Adolescent Medicine, Vienna Health Network, Austria
1Faculty of Medicine, Sigmund Freud Private University, Vienna, Austria
1Department of Pediatrics and Adolescent Medicine, Johannes Kepler University, Linz, Austria
*Corresponding author: Milen Minkov, Professor of Pediatrics, Head, Department of Pediatrics Faculty of Medicine, Sigmund Freud Private University, Vienna, Austria
Submission: February 20, 2024; Published: February 29, 2024

ISSN: 2577-9200 Volume8 Issue1
Abstract
We assessed the pathogen spectrum and resistance of E. coli in 379 consecutive children with positive urine cultures. E. coli was the leading UTI pathogen (84%). It’s resistance to amoxicillin/clavulanic acid increased from 14 to 35% within four years. The rising local resistance of E. coli to amoxicillin/clavulanic acid requires alternative empiric antibiotics for pediatric UTIs.
Keywords:Urinary tract infections; Pathogen; Escherichia coli; Urinary cultures; Antibiotic resistance
Introduction
Urinary tract infection (UTI) is a common bacterial disease characterized by bacterial invasion, adhesion and proliferation in the urogenital tract, associated with local and/or systemic inflammation [1-3]. While most patients experience an uncomplicated course, UTI can cause significant morbidity, including renal scarring, proteinuria, hypertension and renal failure [3]. Early antibiotic therapy (within 72 hours of presentation) is necessary to prevent morbidity and therefore, is started empirically directly after a pathologic urine sample has been obtained [4]. Antibiotic sensitivity of the pathogens depends on both natural and acquired regional resistance [2]. Concern about antimicrobial resistance is growing globally, given rising resistance rates to beta-lactam antibiotics, particularly in Escherichia coli (E. coli) isolates [5]. In the clinical care of children with UTI at our institution, we had the subjective impression that E. coli is increasingly resistant to amoxicillin/clavulanic acid, which has been used as empiric first-line treatment. Therefore, we undertook a retrospective data analysis to evaluate the spectrum of pathogens and the local resistance of E. coli.
Method and Patients
A retrospective data analysis of all patients in the department of pediatrics and adolescent medicine until May 2019, located at Klinik Land strasse and from June 2019 moved to Klinik Floridsdorf was conducted after approval of the local IRB. Included were positive urine cultures in patients (aged one month to 18 years) with clinical signs of a UTI, diagnosed from June 1, 2017, to May 31, 2021. Urine cultures were performed from samples collected by catheterization or clean catch. Pathogen identification and antibiotic resistance data were retrieved from the department of pathology and bacteriology reports. Clinical data were extracted from the electronic patient records. Depending on the patient’s age and the clinical scenario, intravenous or oral antibiotics were given in inpatient or outpatient settings. Patients with a clinically suspected UTI but negative urine culture were excluded.
Result
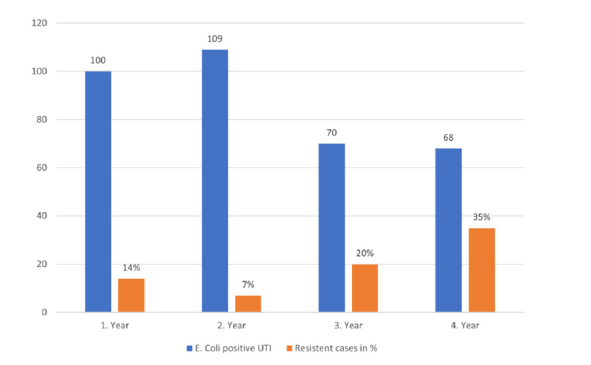
A total of 414 positive urine cultures were performed in 379 patients with suspected UTI, at either primary infection or relapse. The patient cohort comprised 293 female (77%) and 86 male (23%) patients, with a median age of 4.2 years (range, 18 days to 17.9 years). The pathogen spectrum identified in the 414 cultures is presented in Figure 1. The 14% of urine cultures positive for E. coli mixed with one/several pathogens are disputed as contamination but were included in the analysis of antibiotic resistance. Of the 347 cultures positive for E. coli, 287 tested sensitive and 60 resistant to amoxicillin/clavulanic acid. This corresponds to an overall resistance of 17.3%. However, the resistance of E. coli by time (2017-2919 and 2019-2021) showed increasing resistance from 10.5% to 27.5%. Moreover, there was an increasing incidence of E. coli resistance in the Klinik Floridsdorf comparing 2020 and 2021 (Figure 2). At a post hoc evaluation adding data from 2022, this tendency holds on with a resistance rate of 27.6% in 2022 (data not shown). In addition, cultures performed at UTI relapse were analyzed more closely, revealing an E. coli resistance rate to amoxicillin/clavulanic acid of 41%. This percentage significantly surpasses the resistance rate of 27% observed in the entire cohort.
Figure 1:Pathogen spectrum in the studied UTI cohort.
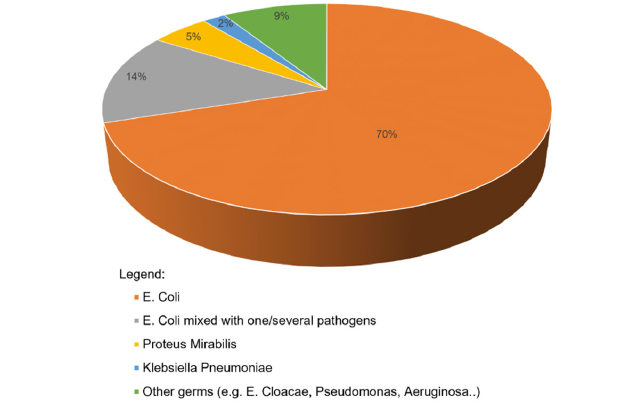
Figure 2:E. coli resistance to amoxicillin/clavulanic acid by year.
Discussion
UTI ranks among the most prevalent bacterial illnesses, with the second-highest incidence in children after otitis media. Concern about antimicrobial resistance is growing globally, given rising resistance rates to beta-lactam antibiotics, particularly in E. coli isolates. In a 2010 retrospective study by Chakupurakal [5], analyzing 547 cases, E. coli’s resistance to amoxicillin/clavulanic acid rose from 0% in 2002 to 48% in 2008 [5]. In a 2017 study by Yakubov [6], covering 829 urine cultures from a single institution, E. coli exhibited a 34% resistance to amoxicillin/clavulanic acid [6]. Likewise, in a 2022 retrospective study by Rosado [7], spanning 2008 to 2019 in a Madrid hospital, E. coli’s resistance to amoxicillin/clavulanic acid increased from 12.2% to 24% [7]. Our data confirm this concerning trend in Austria as well. According to the AURES 2020 Resistance Report recommendations, antibiotics with a resistance rate of >25% should be cautiously used as the likelihood of treatment failure is high [8]. Similarly, the AWMF guidelines “Urinary Tract Infections in Childhood” recommend using antibiotics for empirical therapy with resistance rates for E. coli below 20% [9]. Due to a resistance rate of 35% antibiotics containing the active ingredient amoxicillin/ clavulanic acid are no longer used as the first-line therapy for urinary tract infections in childhood at our institution. Following international guidelines, we implemented second and third generation cephalosporins as the first-line therapy for infants and young children with UTI in our department. From the age of six, therapy with pivmecillinam is used as an alternative drug if the patients can swallow tablets.
Another alarming observation is the even higher resistance of E. coli to amoxicillin/clavulanic acid among children with recurrent UTIs. One explanation is that the broad use of a single antibiotic or a specific class of antibiotics can foster the development of bacterial resistance. Regarding the subgroup of recurrent UTIs, we cannot exclude selection bias, as we could analyze only cases diagnosed and treated in our institution.
To combat the increasing resistance rates effectively, adopting a strategy that involves culturing urine samples and utilizing local antibiograms in hospitals and primary care offices is crucial. This approach would ensure the judicious use of antibiotics with appropriately tailored spectrum.
Conclusion
Our study confirms an increasing local resistance rate of E. coli to amoxicillin/clavulanic acid in a single institution in Austria.
As a result of this analysis, we implemented second and thirdgeneration cephalosporins as the first-line therapy for infants and young children with UTI in our department.
Acknowledgment
None
Transparency Declaration
The authors have no conflicts of interest to disclose.
Funding
No funding of any kind has been received for this work. The data have been generated as part of the routine work of the Department of Pediatrics.
References
- Korbel L, Howell M, Spencer JD (2017) The clinical diagnosis and management of urinary tract infections in children and adolescents. Paediatr Int Child Health 37(4): 273-279.
- Beetz R (2018) Pyelonephritis und urosepsis. Monatsschrift Kinderheilkunde 166(1): 24-32.
- Chang SL, Shortliffe LD (2006) Pediatric urinary tract infections. Pediatr Clin North Am 53(3): 379-400.
- Coulthard MG, Lambert HJ, Vernon SJ, Hunter EW, Keir MJ, et al. (2014) Does prompt treatment of urinary tract infection in preschool children prevent renal scarring: Mixed retrospective and prospective audits. Arch Dis Child 99(4): 342-347.
- Chakupurakal R, Ahmed M, Sobithadevi DN, Chinnappan S, Reynolds T (2010) Urinary tract pathogens and resistance pattern. J Clin Pathol 63(7): 652-654.
- Yakubov R, Vanden AM, Machamad K, Amit H, Erez N, et al. (2017) Antimicrobial resistance among uropathogens that cause childhood community-acquired urinary tract infections in central Israel. Pediatr Infect Dis J 36(1): 113-115.
- Rosado MR, Molina AG, Velasco AL, Gloria CC, Paula VL, et al. (2022) Urinary tract infection in pediatrics: Study of uropathogens and their resistance in a Madrid hospital. Arch Esp Urol 75(9): 791-797.
- (2021) Federal ministry for social affairs, health, care and consumer protection (BMSGPK); Resistance report Austria-AURES.
- (2021) Society for pediatric nephrology and working group on child and adolescent urology of the German society for urology: Interdisciplinary S2k guidelines: Urinary tract infections in children: Diagnostics, therapy and prophylaxis.
© 2024 Milen Minkov. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






