- Submissions

Full Text
Research in Pediatrics & Neonatology
Cetirizine in Children with Coexisting Allergic Rhinitis and Asthma: A Randomized Trial
Jingyang Li1, Guiying Ruan2, Hong Wang3, Linxiu Tu4, Xiaoqun Jin5, Yanming Lu6,Yaping Chen7, Yihui Yang8 and Yixiao Bao9*
1Department of Pediatric Respirology Medicine, Shanghai Jiao Tong University School of Medicine, Shanghai 200127, China
2Department of Paediatrics, Taizhou Women and Children Hospital affiliated to Wenzhou Medical University, China
3Department of Paediatrics, Civil Aviation General Hospital, China
4Department of Paediatrics, The People’s Hospital of Beilun District, Beilun Branch Hospital of The First Affiliated Hospital of Zhejiang University School of Medicine, China
5Department of Paediatrics, Shanghai Putuo District People’s Hospital, China
6Department of Paediatrics, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, China
7Department of Paediatrics, Ningbo Women and Children’s Hospital, China
8Department of Otolaryngology, Ningbo Women and Children’s Hospital, China
9Department of Pediatrics, Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine, China
*Corresponding author: Yixiao Bao, Department of Pediatrics, Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai 200127, China
Submission: August 15, 2023; Published: September 07, 2023

ISSN: 2577-9200 Volume7 Issue5
Abstract
Asthma and allergic rhinitis often coexist and it is suggested that the second-generation H1 antihistamines may be a promising treatment for patients with allergic rhinitis and asthma. This study was conducted to investigate the efficacy and safety of oral cetirizine in children with coexisting allergic rhinitis and asthma. 552 children, mean age 4.8 years, were randomized to receive daily oral cetirizine dihydrochloride drops plus inhaled corticosteroids (intervention group) or inhaled corticosteroids alone (control group) for 12 weeks. Allergic rhinitis symptoms, recent asthma control, the occurrence of acute asthma attacks, use of rescue medications, and adverse events were recorded. The proportions of subjects with good asthma control in the intervention group were 90.5%, 90.7% and 95.6% at Weeks 12, 16 and 24, respectively, which were significantly higher than the proportions in the control group (61.3%, 68.5% and 78.2%, respectively). The proportions of subjects with asthma attacks were significantly lower in the intervention group than the control group at Weeks 12 and 16. The use of rescue medications was significantly less in the intervention group than in the control group. Nasal symptoms improved in both groups and the improvements were significantly more pronounced in the intervention group. Treatment with cetirizine in addition to inhaled corticosteroids significantly improved asthma control and reduced nasal symptoms in children compared to inhaled corticosteroids alone.
Keywords:Allergic rhinitis; Asthma; Cetirizine; Children; Randomized trial
Introduction
Asthma and Allergic Rhinitis (AR) are the two most common chronic airway disorders in children and adolescents. Studies have shown that between 60-80% of children with asthma also have AR and 20-38% of patients with AR have symptoms of asthma [1,2]. In China, depending on the regions, AR is found in 35-50% of children with asthma and asthmatic symptoms are experienced by 10-18% of children with AR [3]. The coexistence of these two conditions appears to be more common in younger than in older patients. AR has a significant impact on asthma. Uncontrolled AR is associated with more severe and more difficult to control asthma and substantially impaired quality of life [4,5]. AR is also a risk factor for asthma [6,7]. In up to 30% of patients with AR who have no previous history of asthma, the provocative bronchial challenge with methacholine can elicit bronchial hyperreactivity [8]. AR and asthma share similar allergic inflammatory mechanisms and may represent two manifestations of the same chronic allergic respiratory syndrome [9,10]. To emphasize the link of allergic disorder between the upper and lower respiratory tracks, a term called ‘Combined Allergic Rhinitis and Asthma Syndrome’ was proposed by the World Allergy Organization [11].
It has therefore, been suggested by the Allergic Rhinitis and its Impact on Asthma (ARIA) initiative that control of AR may improve symptoms of coexisting asthma [7,12]. The ARIA initiative in 2010 recommended that the second-generation H1 antihistamines may be a promising treatment for patients with AR and asthma [13]. Cetirizine is a second-generation H1-receptor antagonist that does not cause sedation or interact with cytochrome P450. A few randomized controlled trials have shown that cetirizine is safe and effective in relieving both upper and lower respiratory tract symptoms in patients with coexisting AR and asthma [14-16]. Previous studies were mostly conducted in adolescents and adults. Clinical trials on the efficacy and safety of cetirizine on asthma control in children with asthma and AR are needed to test the hypothesis that effective control of AR could lead to better control of asthma symptoms. This randomized trial aimed to investigate the efficacy and safety of cetirizine dihydrochloride drops on asthma and AR outcomes in children with coexisting AR and asthma receiving inhaled corticosteroids.
Method and Materials
Trial design and patients
This was a multicenter, open-label, randomized trial conducted between October 2015 and July 2017 in 18 hospitals from Beijing, Shanghai and Zhejiang in China. The study was approved by the Ethics Committee of Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (No. XHEC-D-2020-034) and the study was in accordance with the Declaration of Helsinki. Informed consent was taken from all individual participants and their parents. Eligible study participants were children aged from 6 months to 12 years old. Inclusion criteria were: a) a diagnosis of asthma according to the Guideline for the diagnosis and optimal management of asthma in children in China [17]; b) mild persistent and moderate persistent asthma according to Global Initiative for Asthma Classification of Asthma based on the severity of clinical features [18]; c) a concomitant diagnosis of AR according to the Guidelines for the Diagnosis and Treatment of Allergic Rhinitis in Children in China [19]; d) current regular use of inhaled corticosteroids for at least one month. Exclusion criteria were: 1) severe cardiovascular diseases, liver and kidney dysfunction, or diseases of the hematopoietic system; 2) severe chronic obstructive pulmonary disease, bronchiectasis, congestive heart failure, tuberculosis, or nasal ventilatory dysfunction caused by nasal septum deviation and nasal polyps; 3) allergic to the medication under investigation or hydroxyzine; 4) participation in other studies within one month before enrolment; 5) use of cetirizine, other kinds of antihistamines, leukotriene receptor antagonists, or immunotherapy in the previous month; 6) considered as unable to complete the trial or as an unsuitable subject for other reasons at the discretion of the study investigators.
Randomization
An independent statistician generated a randomization list using Stata, with random permuted blocks of random size stratified by recruitment centre in a 2: 1 (intervention group vs control group) ratio. At enrollment, the sequence was concealed from investigators and research staff who confirmed consent and eligibility before allocation was revealed. The 2: 1 ratio randomization was chosen because the subjects included in this study were seeking medical attention because of unsatisfactory outcomes when taking inhaled corticosteroids alone so it was considered appropriate to provide active treatment for the majority of subjects. Unfortunately, it was not possible to provide a placebo oral solution for the control group.
Study protocol and interventions
Children in the intervention group received oral cetirizine dihydrochloride drops (Bright Future Pharmaceutical Laboratories Ltd., Hong Kong) and inhaled corticosteroids and those in the control group received inhaled corticosteroids alone. The dosage of cetirizine dihydrochloride drops (10mg/ml) was as follows: a) 0.25mg/kg twice a day for 6 months to one year old; b) 0.25ml (2.5mg) twice a day for one to two years old; c) 0.5ml (5mg) daily for two to six years old; d) 1ml (10mg) daily for six to 12 years old. The most common choice of inhaled corticosteroids included fluticasone propionate aerosol, beclomethasone propionate aerosol, and budesonide aerosol. During the study period, children were allowed to use inhaled short-acting β-receptor agonists (SABAs) to relieve acute exacerbation of asthma or systemic corticosteroids for severe symptoms. The treatment period was 12 weeks, followed by a 12-week follow-up period. Study visits were at baseline, Week 4, Week 12, Week 16 and Week 24.
Data collection and study outcomes
Data collection was performed at each study visit. Patients’ age, sex, duration of AR, and duration of asthma were recorded at baseline. Nasal symptoms of AR, including sneezing, runny nose, congestion, and itching were evaluated using a 0-10 visual analogue scale (VAS), with 0 indicating no symptom and 10 most severe symptoms. Mild symptoms referred to those of VAS from 0 to 3. Recent asthma control was based on symptoms over the previous 4 weeks [18]. A patient was considered in good control when all four of the following criteria were fulfilled: a) daytime symptoms ≤2 days per week, lasting only a few minutes and rapidly relieved by rapid-acting bronchodilator; b) no limitation of activities; c) no symptoms during night or when waked up; d) need for SABA ≤2 days per week. Partial control referred to any of the following: a) daytime symptoms >2 days per week; b) any limitation of activities; c) any symptoms during night or when waked up; d) need for SABA >2 days per week. Poor control referred to meeting ≥3 features of partial control within the same week. The occurrence of an acute asthma attack(s) and frequency of use of inhaled SABAs and systemic corticosteroids for acute exacerbation of asthma during the study period was recorded. An acute asthma attack referred to sudden onset of symptoms such as wheezing, shortness of breath, coughing, or chest tightness, or exacerbation of the original symptoms, accompanied by dyspnea, or reduced flow of exhalation. Adverse events during the study period were observed and recorded by the treating physicians.
Statistical analyses and sample size calculation
Statistical analyses were performed using IBM Statistics Package for Social Science (SPSS 22.0). Analyses were performed on an intention-to-treat basis. Demographic and safety data were summarized using descriptive statistics. Comparisons of baseline characteristics between the two groups were performed using Student’s t-test or chi-square test, depending on the distribution. Continuous variables were analyzed using analysis of covariance models, with terms for the treatment group, the study cites, and baseline as covariates. Categorical variables were analyzed using Cochran-Mantel-Haenszel tests stratified by study site. All hypotheses were two-tailed and a p-value <0.05 was considered statistically significant. The sample size was calculated with α = 0.05, β = 0.80, Nt: Nc = 2:1, Δ = 0.1, based on the different disease evaluation index, such as asthma control, and effect size in a previous study [16]. The sample size estimation ranged from 234 to 915. It was expected to include at least 500 patients in the analysis and in this study, a total of 900 patients were screened and 552 were finally included in the study.
Result
Characteristics of study subjects
Figure 1 shows the CONSORT flow chart of the study. A total of 552 children were enrolled. Four subjects were excluded from analyses, including one in the intervention group who did not use the prescribed cetirizine and three in the control group who were ineligible but mistakenly randomized. The final analyses included 548 children, 367 in the interventional group and 181 in the control group. Table 1 shows the baseline characteristics of the two groups. The two groups were comparable in terms of sex, age, body weight, height, duration of AR and asthma symptoms, and VAS scores of AR symptoms. However, the proportion of children with mild persistent asthma at baseline was significantly higher in the control group than in the interventional group (88.4% vs. 79.0%, p=0.005). This difference would weaken the advantage of intervention group to some extent, which was supported by subgroup analysis.
Figure 1:CONSORT diagram of the study.
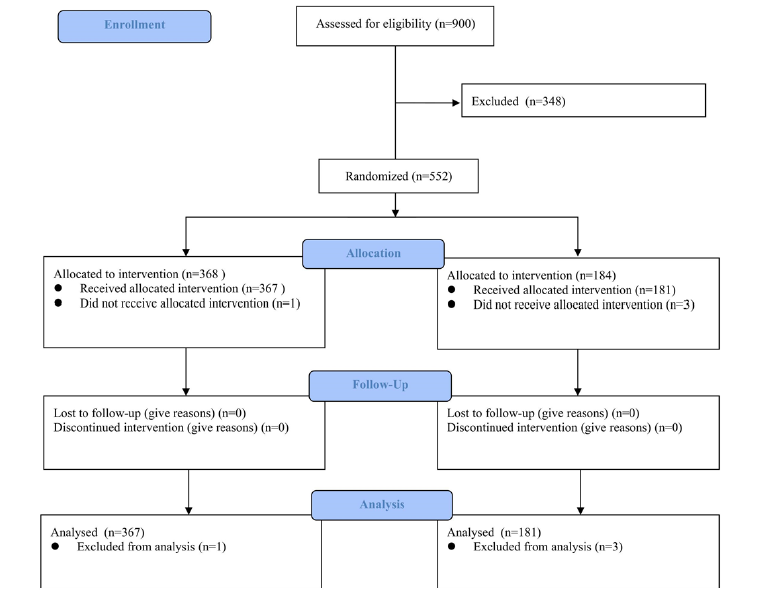
Table 1:Baseline characteristics of the study patients.
Results are mean ± standard deviation (SD) or number (%) unless otherwise indicated. AR: Allergic Rhinitis; C-ACT:
Children-Asthma Control Test; VAS: Visual Analogue Scale.
Asthma control
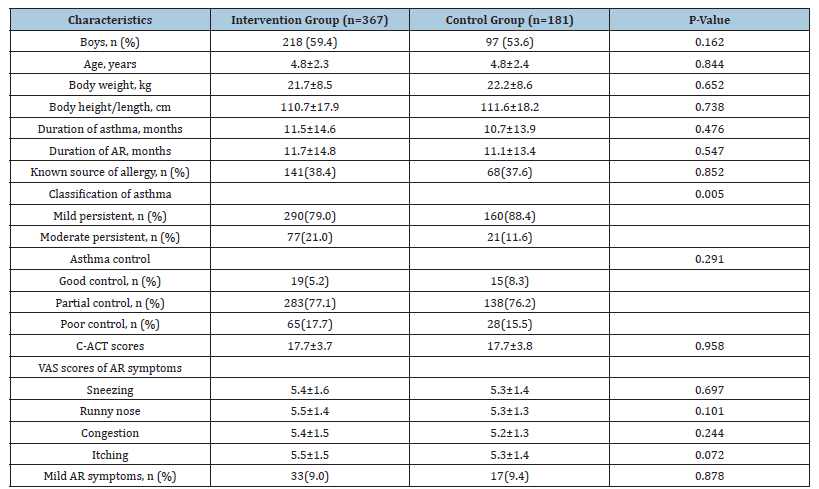
Figure 2:Changes of proportions of children with good control (grey), partial control (pattern), and poor control (white) of the two groups.
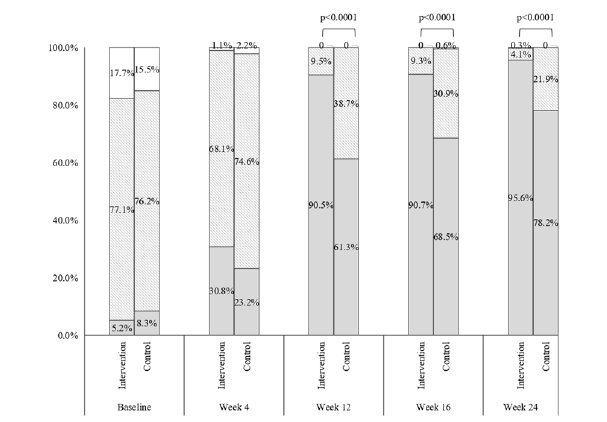
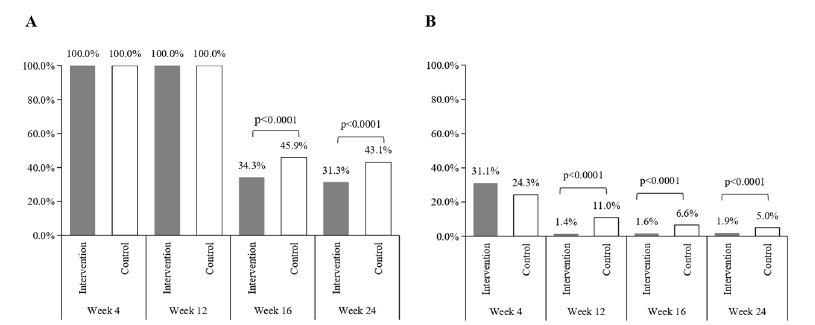
The proportion of subjects with good control increased and that of poor control decreased in both groups after randomized treatment. Although there was no significant difference in categories of recent asthma control between the two groups at Week 4 (p= 0.121), the proportion with good control in the intervention group was greater than 90% at Weeks 12, 16 and 24 (90.5%, 90.7%, and 95.6%, respectively) and these were significantly higher than the values in the control group (61.3%, 68.5% and 78.2%, respectively, all p<0.0001) (Figure 2). At baseline, all children had experienced acute asthma attacks recently. After systematic treatment, the proportion of subjects with asthma attacks decreased sharply in both groups at Week 4 (32.4% in the intervention group and 23.2% in the control group, p=0.026). And the proportion with asthma attacks was numerically less in the intervention than in the control group at Weeks 12 (1.6 % vs. 13.3%), 16 (1.4% vs. 6.1%) and 24 (1.9% vs. 4.4%), with differences at Weeks 12 and 16 being statistically significant (p<0.0001 and <0.01, respectively) (Figure 3). Use of inhaled corticosteroids was required by all children during the treatment period until Week 16 (Figure 4A). Only 34.3% and 45.9% of children in the intervention and control group, respectively, required the use of inhaled corticosteroids at Week 16. The corresponding figures at Week 24 were 31.3% and 43.1%, respectively. Significant between-group differences were found at Week 16 and 24 (both p <0.0001). The use of SABAs was comparable between the two groups at Week 4 but was significantly less in the intervention group than in the control group at Weeks 12, 16 and 24 (all p <0.0001) (Figure 4B).
Figure 3:Proportions of children with asthma attacks in the interventional group (grey bar) and control group (white bar).
Figure 4:Proportions of using inhaled corticosteroids (A) and inhaled rapid-acting β-receptor agonists (B) during the study period in the interventional group (grey bar) and control group (white bar).
AR symptoms
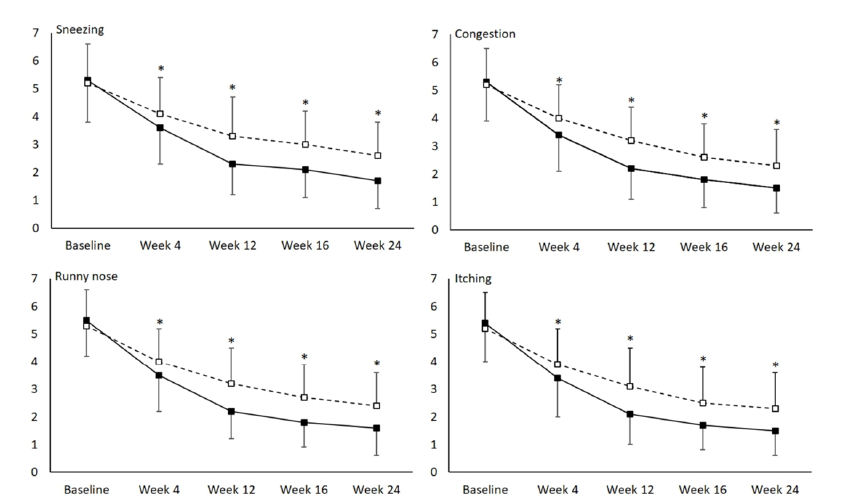
Figure 5 shows changes in VAS scores of AR symptoms for both groups. Symptoms of sneezing, runny nose, congestion and itching improved during the treatment period and follow-up period and such improvements were significantly more pronounced in the intervention group than in the control group, reflected by the significantly lower VAS scores at all follow-up visits in the intervention group (all p <0.0001).
Figure 5:Changes of visual analogue scale scores of four allergic rhinitis symptoms - sneezing, congestion, runny nose and itching-during the study period in the intervention group (solid line) and control group (dashed line). *p <0.0001 comparing between the two groups.
Adverse events
There were only two adverse events during the study period, both of which occurred in the intervention group and were accidental falls that were deemed unrelated to the study medications. There were no adverse events noted of sedation, decreasing of attention and cognition, urinary retention, intestinal constipation, or hypotension.
Discussion
In this large-randomized trial, daily use of cetirizine dihydrochloride drops significantly improved asthma control and reduced asthma attacks and use of inhaled corticosteroids and SABAs in children between 6 months and 12 years old. These effects were accompanied by improved control of AR symptoms. Cetirizine was well tolerated with no significant adverse events noted. These results demonstrated the efficacy and lack of adverse effects of cetirizine in children with AR and concomitant asthma and provided evidence to support the potential utility of second-generation H1- antagonists as a complementary treatment in the comprehensive management of these children. These findings further support the theory of a combined allergic respiratory syndrome [20]. Several large epidemiology studies have found a higher prevalence of rhinitis in asthma [21], whether allergic or non-allergic rhinitis, and that the presence of rhinitis increases the risk for development of asthma [6,7] and leads to a less favorable evolution in patients with asthma [22]. Apart from the epidemiology studies, it is postulated that the upper and lower airways function as a unit, and disease processes might be interrelated. Bronchial hyperresponsiveness and heightened reactivity to a variety of stimuli are shared physiology of AR and asthma [23,24]. The immunopathology of AR is also similar to the TH2-type immune response and eosinophilic inflammation in asthma [25].
On the therapeutic level of clinical evidence, studies have shown that effective control of AR was beneficial to reducing asthma symptoms [26], emergency room visits [27], primary care visits [28], hospitalization [28,29] and the severity of bronchial hyperresponsiveness [30]. Intranasal corticosteroids are considered the most effective drug for the pharmacologic management of AR and have been shown in a few studies to reduce asthma symptoms and bronchial hyperresponsiveness in patients with coexisting AR and asthma [30-34]. However, these studies were mostly conducted in a small study cohort. A meta-analysis by Taramarcaz [5] in 2003 summarized results of 14 trials involving 477 patients and showed that intranasal corticosteroids failed to achieve a statistically significant effect in improving asthma outcomes or hyperresponsiveness, despite a trend of favoring a beneficial effect [5]. A more recent meta-analysis by Lohia et al. [26] in 2013 performed several subgroup analyses [26]. Their findings showed that intranasal corticosteroids offered no additional beneficial effect when patients were already treated with orally inhaled corticosteroids but could significantly improve several asthma outcomes when patients were not on orally inhaled corticosteroids, or when corticosteroids were inhaled through the nose into the lungs.
Most of these previous studies were conducted in an adult cohort or a mixed cohort of adults and adolescents. Our large clinical trial provided additional clinical evidence to support the concept of integrating the management of AR in asthma control in children. Antihistamines can be used as add-on therapy to improve efficacy and reduce glucocorticoid use. A small randomized controlled trial of 12 adult patients demonstrated a protective nasal effect of cetirizine against bronchial hyperresponsiveness measured six hours after nasal allergen challenge in patients with AR [35]. Cetirizine has been shown to significantly reduce symptoms of pollen-associated asthma [36,37]. Two large randomized controlled trials in patients with concomitant seasonal AR and asthma aged 12 years or older showed that cetirizine could not only significantly relieve AR symptoms but also improve asthma symptoms compared with placebo [14,16]. Our findings are consistent with theirs by showing that cetirizine resulted in better asthma control and less use of rescue medications in children.
In this study, cetirizine exerted a significant effect in the treatment of asthma and AR and the underlying mechanism might be related to its antihistamine and anti-inflammatory effects. Histamine is an important mast cell and basophil-derived mediator in allergic diseases, which can cause smooth muscle contraction and increased vascular permeability leading to mucosal oedema. For asthma, histamine concentrations are higher in bronchoalveolar lavage as a result of increased peripheral basophil and mast cell degranulation, which contribute to airway hyperreactivity. It has been well demonstrated that the antihistamine cetirizine is very effective in histamine-induced bronchoconstriction [38]. Cetirizine is also a first-line treatment for AR because of its strong antihistamine effect. Furthermore, cetirizine has an anti-inflammatory effect that cannot be entirely explained by antagonism of the H1 receptor [39]. Cetirizine could inhibit antigeninduced eosinophil recruitment in the nose and lung, protect against the asthmatic late-phase reaction and decrease eosinophil and neutrophil infiltration and eosinophil cationic protein and eosinophil peroxidase in nasal lavage from patients with allergic rhinitis [39,40]. A recent meta-analysis performed that cetirizine could improve clinical improvement and quality of life in children with AR and is well tolerated in the pediatric population [41]. In a previous study, the therapeutic effect of cetirizine, nasal fluticasone and the combination of cetirizine and nasal fluticasone were compared in AR patients [42]. The study showed that patients who received the combination treatment achieved the best results on all symptoms including obstruction, which continued even after interrupting the treatment with fluticasone. This suggested there may be a synergistic action of cetirizine and the corticosteroid [42]. In the present study, patients in the intervention group required less ICS and achieved better disease control, which also suggests a synergistic effect of cetirizine with ICS and this may be related to the antihistamine effect and anti-inflammatory effect of cetirizine. Further study is needed to investigate the underlying mechanisms of the synergistic effect between antihistamine and ICS in asthma.
This study has several limitations. Bias might arise because the study adopted an open-label design, lacking a placebo and objective outcome measures. The age of the subjects ranged from 6 months to 12 years, and there is some heterogeneity in disease severity or clinical manifestations of different ages. For example, transient wheezing and infections are more common in the younger group, which may cause bias. In addition, the results may be influenced by some confounding factors. For example, the proportion of subjects with acute asthma attack decreased sharply in both groups after four weeks of treatment. Levels of asthma control also improved significantly in both groups in week 4. These improvements might reflect a natural course of disease in patients with mild persistent and moderate persistent asthma. Meanwhile, most patients visit at baseline due to clinical progression, which may be related to seasonal effect or substandard medication. The improvements in week 4 may be attributed more to systematic treatment, while the advantage of cetirizine emerged during the long-term treatment phase. An additional limitation is that we did not measure the exact dosage change of inhaled corticosteroids. We used recent asthma control as the primary outcome to reflect the overall severity and/or activity of the disease. Two important outcomes were not assessed: Asthma-related quality of life and pulmonary function. In a four-week randomized placebo-controlled study, Nathan et al. [16] showed that patients treated with cetirizine had significantly greater improvements in the Asthma Quality of Life Questionnaire [16]. Studies have shown that treatment with cetirizine was not associated with significant changes in pulmonary function tests [14-16]. Nonetheless, we included the occurrence of an asthma attack as one of the outcomes and the results showed a significant reduction in the risk of an acute asthma attack in children treated with cetirizine.
Conclusion
In conclusion, this study found that daily cetirizine dihydrochloride significantly reduced symptoms of AR and asthma, improved asthma control and reduced asthma attacks and use of rescue medications in children aged between 6 months and 12 years suffering from concomitant AR and asthma. The collective evidence from this study and previous ones corroborates the importance of assessing and treating AR in asthmatic patients. Second-generation H1-antagonists could be considered as a complementary treatment in the comprehensive management of patients with AR and asthma.
Acknowledgement
The authors would like to thank Ling Cao (Capital Institute of Pediatrics, Beijing, China) and Changchong Li (The 2nd Affiliated Hospital & Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China) for their guidance. Furthermore, the authors also warmly thank all the centers participating in the study: Tiefeng Wu: Wenzhou Hospital of Integrated Traditional Chinese and Western Medicine, Wenzhou (China). Feng Chen: Hospital of China Metallurgical Corporation, Shanghai (China). Hengtao Li: Fengcheng Hospital in Fengxian District, Shanghai (China). Juan Kang: No. 3 People Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai (China). Lieping Huang: Zhoushan Hospital affiliated to Wenzhou Medical University, Zhoushan (China). Lijuan Bai: Miyun County Hospital, Beijing (China). Ping Wen: Shanghai Seventh People’s Hospital, Shanghai (China). Tingfu Zhu: Li Huili Hospital of Ningbo Medical Center, Ningbo (China). Wei Dong: Nanxiang Hospital of Shanghai Jiading District, Shanghai (China). Zhifei Li: The Affiliated Hospital of Medical School of Ningbo University, Ningbo (China). Zongrong Yan: Liangxiang Hospital, Fangshan District, Beijing (China).
Compliance with Ethics Guideline
Jingyang Li, Guiying Ruan, Hong Wang, Linxiu Tu, Xiaoqun Jin, Yanming Lu, Yaping Chen, Yihui Yang and Yixiao Bao declare that they have no conflict of interest. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Conflict of Interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
References
- Grossman J (1997) One airway, one disease. Chest 111(2 Suppl): 11S-16S.
- Pawankar R, Bunnag C, Khaltaev N, Bousquet J (2012) Allergic rhinitis and its impact on asthma in Asia pacific and the ARIA update 2008. World Allergy Organ J 5(Suppl 3): S212-S217.
- Zhao J, Bai J, Shen K, Xiang L, Huang S, et al. (2010) Self-reported prevalence of childhood allergic diseases in three cities of China: a multicenter study. BMC Public Health 10: 551.
- Corren J, Adinoff AD, Buchmeier AD, Irvin CG (1992) Nasal beclomethasone prevents the seasonal increase in bronchial responsiveness in patients with allergic rhinitis and asthma. J Allergy Clin Immunol 90(2): 250-256.
- Taramarcaz P, Gibson PG (2003) Intranasal corticosteroids for asthma control in people with coexisting asthma and rhinitis. Cochrane Database Syst Rev 2003(4): CD003570.
- Settipane RJ, Hagy GW, Settipane GA (1994) Long-term risk factors for developing asthma and allergic rhinitis: A 23-year follow-up study of college students. Allergy Proc 15(1): 21-25.
- Shaaban R, Zureik M, Soussan D, Neukirch C, Heinrich J, et al. (2008) Rhinitis and onset of asthma: A longitudinal population-based study. Lancet 372(9643): 1049-1057.
- Fireman P (2000) Rhinitis and asthma connection: management of coexisting upper airway allergic diseases and asthma. Allergy Asthma Proc 21(1): 45-54.
- Togias A (2003) Rhinitis and asthma: Evidence for respiratory system integration. J Allergy Clin Immunol 111(6): 1171-1183.
- Togias A, Gergen PJ, Hu JW, Babineau DC, Wood RA, et al. (2019) Rhinitis in children and adolescents with asthma: Ubiquitous, difficult to control, and associated with asthma outcomes. J Allergy Clin Immunol 143(3): 1003-1011.e10.
- Paiva FLKD, Paiva FLAM, Monteiro TM, Bezerra GC, Bernardo LR, et al. (2019) Combined allergic rhinitis and asthma syndrome (CARAS). Int Immunopharmacol 74: 105718.
- Bousquet J, Schunemann HJ, Samolinski B, Demoly P, Baena CCE, et al. (2012) Allergic rhinitis and its impact on asthma (aria): Achievements in 10 years and future needs. J Allergy Clin Immunol 130(5): 1049-1062.
- Brozek JL, Bousquet J, Agache I, Agarwal A, Bachert C, et al. (2017) Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol 140(4): 950-958.
- Grant JA, Nicodemus CF, Findlay SR, Glovsky MM, Grossman J, et al. (1995) Cetirizine in patients with seasonal rhinitis and concomitant asthma: Prospective, randomized, placebo-controlled trial. J Allergy Clin Immunol 95(5 Pt 1): 923-932.
- Aaronson DW (1996) Evaluation of cetirizine in patients with allergic rhinitis and perennial asthma. Ann Allergy Asthma Immunol 76(5): 440-446.
- Nathan RA, Finn AF, LaForce C, Ratner P, Chapman D, et al. (2006) Comparison of cetirizine-pseudoephedrine and placebo in patients with seasonal allergic rhinitis and concomitant mild-to-moderate asthma: Randomized, double-blind study. Ann Allergy Asthma Immunol 97(3): 389-396.
- (2008) Guidelines for the diagnosis and prevention of bronchial asthma in children. Chinese Journal of Pediatrics 46(10): 745-753.
- Koshak EA (2007) Classification of asthma according to revised 2006 GINA: Evolution from severity to control. Ann Thorac Med 2(2): 45-46.
- (2011) Guidelines for the diagnosis and treatment of allergic rhinitis in children (2010, Chongqing). Chinese journal of otolaryngology head and neck surgery 46(1): 7-8.
- Giavina BP, Aun MV, Takejima P, Kalil J, Agondi RC (2016) United airway disease: Current perspectives. J Asthma Allergy 9 :93-100.
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, et al. (2008) Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and Aller Gen). Allergy 63 Suppl 86: 8-160.
- Greisner WA, Settipane RJ, Settipane GA (2002) The course of asthma parallels that of allergic rhinitis: A 23-year follow-up study of college students. Allergy Asthma Proc 21(6): 371-375.
- Khan DA (2014) Allergic rhinitis and asthma: epidemiology and common pathophysiology. Allergy Asthma Proc 35(5): 357-361.
- Lambrecht BN, Hammad H (2015) The immunology of asthma. Nat Immunol 16(1): 45-56.
- Okano M, Kariya S, Ohta N, Imoto Y, Fujieda S, et al. (2015) Association and management of eosinophilic inflammation in upper and lower airways. Allergol Int 64(2): 131-138.
- Lohia S, Schlosser RJ, Soler ZM (2013) Impact of intranasal corticosteroids on asthma outcomes in allergic rhinitis: A meta-analysis. Allergy 68(5): 569-579.
- Bousquet J, Gaugris S, Kocevar VS, Zhang Q, Yin DD, et al. (2005) Increased risk of asthma attacks and emergency visits among asthma patients with allergic rhinitis: A subgroup analysis of the investigation of montelukast as a partner agent for complementary therapy [corrected]. Clin Exp Allergy 35(6): 723-727.
- Thomas M, Kocevar VS, Zhang Q, Yin DD, Price D (2005) Asthma-related health care resource use among asthmatic children with and without concomitant allergic rhinitis. Pediatrics 115(1): 129-134.
- Crystal PJ, Neslusan C, Crown WH, Torres A (2002) Treating allergic rhinitis in patients with comorbid asthma: The risk of asthma-related hospitalizations and emergency department visits. J Allergy Clin Immunol 109(1): 57-62.
- Agondi RC, Machado ML, Kalil J, Giavina BP (2008) Intranasal corticosteroid administration reduces nonspecific bronchial hyperresponsiveness and improves asthma symptoms. J Asthma 45(9): 754-757.
- Camargos PA, Rodrigues ME, Lasmar LM (2004) Simultaneous treatment of asthma and allergic rhinitis. Pediatr Pulmonol 38(3): 186-192.
- Pedersen B, Dahl R, Lindqvist N, Mygind N (1990) Nasal inhalation of the glucocorticoid budesonide from a spacer for the treatment of patients with pollen rhinitis and asthma. Allergy 45(6): 451-456.
- Pedersen W, Hjuler I, Bisgaard H, Mygind N (1998) Nasal inhalation of budesonide from a spacer in children with perennial rhinitis and asthma. Allergy 53(4): 383-387.
- Camargos P, Ibiapina C, Lasmar L, Cruz AA (2007) Obtaining concomitant control of allergic rhinitis and asthma with a nasally inhaled corticosteroid. Allergy 62(3): 310-316.
- Aubier M, Neukirch C, Peiffer C, Melac M (2001) Effect of cetirizine on bronchial hyperresponsiveness in patients with seasonal allergic rhinitis and asthma. Allergy 56(1): 35-42.
- Kurzeja A, Riedelsheimer B, Hulhoven R, Bernheim J (1989) Cetirizine in pollen-associated asthma. Lancet 1(8637): 556.
- Dijkman JH, Hekking PR, Molkenboer JF, Nierop G, Vanderschueren R, et al. (1990) Prophylactic treatment of grass pollen-induced asthma with cetirizine. Clin Exp Allergy 20(5): 483-490.
- Walsh GM (2002) Second-Generation Antihistamines in Asthma Therapy. American Journal of Respiratory Medicine 1(1): 27-34.
- Walsh GM, Annunziato L, Frossard N, Knol K, Levander S, et al. (2001) New insights into the second generation antihistamines. Drugs 61(2): 207-236.
- Parisi GF, Leonardi S, Ciprandi G, Corsico A, Licari A, et al. (2020) Cetirizine use in childhood: An update of a friendly 30-year drug. Clin Mol Allergy 18: 2.
- Zhou P, Jia Q, Wang Z, Zhao R, Zhou W (2022) Cetirizine for the treatment of allergic diseases in children: A systematic review and meta-analysis. Front Pediatr 10: 940213.
- D'Ambrosio FP, Gangemi S, Merendino RA, Arena A, Ricciardi L, et al. (1998) Comparative study between fluticasone propionate and cetirizine in the treatment of allergic rhinitis. Allergol Immunopathol (Madr) 26(6): 277-282.
© 2023 Yixiao Bao. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






