- Submissions

Full Text
Research in Pediatrics & Neonatology
Interstitial Lung Disease and Periventricular Nodular Heterotopia on Imaging in a Young Child: Think Filamin A Mutation
Livja Mertiri1*, Gunes Orman1, Javeed Akhter2, Silvana Molossi3, Stephen F Kralik1 and Thierry A G M Huisman1
1Edward B. Singleton Department of Radiology, Texas Children’s Hospital and Baylor College of Medicine, Houston, TX, USA
2Advocate Children’s Hospital, Oak Lawn, IL, USA
3Division of Cardiology, Department of Pediatrics, Baylor College of Medicine, Texas Children’s Hospital, Houston, Texas, USA
*Corresponding author: Livja Mertiri, Edward B Singleton Department of Radiology, Texas Children’s Hospital and Baylor College of Medicine, 6701 Fannin Street, Suite 470, Houston, Texas, 77030, USA
Submission: July 20, 2023; Published: August 01, 2023

ISSN: 2577-9200 Volume7 Issue5
Abstract
Filamin A is an actin linking protein that has an important role in building the internal network of protein filaments in the cell cytoskeleton. It is involved in cell migration, proliferation and differentiation and plays consequently a key role in the development of multiple organs. Mutations of the filamin A gene affect the brain, heart, connective tissue, skeleton, and blood vessels leading to heterogeneous phenotypes. The variability of clinical presentation appears challenging for an early and correct diagnosis. We present a 12-year-old girl with interstitial lung disease and periventricular nodular heterotopia who was diagnosed with filamin A mutation to discuss the imaging findings in order to familiarize radiologists with this rare multi-system genetic disorder. The aim of this report is to alert radiologists and neuroradiologists that when faced with the combination of interstitial lung disease and periventricular nodular heterotopia in a child, they should suspect filamin A mutations, and a targeted genetic work up should be performed for a definitive diagnosis confirmation.
Keywords: Filamin A mutation; Interstitial lung disease; Periventricular nodular heterotopia; Patent ductus arteriosus; Filamin A deficiency; Pediatric brain MRI
Abbreviations:CT: Computed Tomography; DEXA: Dual Energy X-ray Absorptiometry; FLNA: Filamin A; GOF: Gain Of Function; ILD: Interstitial Lung Disease; LAM: Lymphangioleiomyomatosis; LOF: Loss Of Function; OPD-SD: Oto-Palato-Digital Spectrum Disorders MRI: Magnetic Resonance Imaging; PDA: Patent Ductus Arteriosus; PNH: Periventricular Nodular Heterotopia
Introduction
Filamin A (FLNA) is a cytoskeleton-associated gene located on chromosome Xq28 and encodes for the protein filamin A. Filamin A is essential for the building of the cytoskeleton of the cells throughout the body. In particular, filamin A binds to the protein actin allowing for the complex internal network of protein filaments that represents the internal cellular cytoskeleton. Filamin A links the cellular membrane proteins, integrins and second messengers. It regulates cell morphology, adhesion, and migration. Furthermore, Filamin A is involved in intracellular signal transduction, transport, and cell proliferation and differentiation [1]. Filamin A plays consequently a key role in the development of the brain, heart, connective tissue, skeleton, and blood vessels [2]. Mutations of the FLNA gene may cause a wide range of diseases affecting multiple organs. There is no known association between the phenotype and the genetic variants [3]. In fact, several infants with interstitial lung disease (ILD) inherited FLNA mutations from their mothers who, conversely, had no apparent lung involvement [2,4]. The phenotypic variation may be determined by the X chromosome inactivation level in females and the severity of the mutation in both sexes [2]. Mutations in the FLNA gene can be both secondary to loss of function (LOF) and gain of function (GOF).
The clinical presentation usually associated with LOF mutations includes periventricular nodular heterotopia (PNH) [2], cardiac abnormalities, thrombocytopenia [5], ILD and short bowel disease [6], whereas GOF mutations are usually associated with skeletal dysplasia, such as fronto-metaphyseal dysplasia, Melnick-Needles syndrome, or Oto-palato-digital spectrum disorders (OPD-SD) [2]. Filamin A mutation related pathology has been recognized over the last two decades [7]. Neurological, cardiovascular, and skeletal symptoms may dominate the clinical presentation [2]. However, recently, the respiratory phenotype has been recognized as a clinical consequence of FLNA loss of function mutation [1]. Symptoms usually occur during early childhood [8] and due to the heterogeneity of the diseases and the involvement of multiple organs, imaging plays a key role in the diagnosis. Goal of this manuscript is to present and discuss the imaging findings in a 12-year-old girl with confirmed FLNA mutation in order to familiarize and alert radiologists about this rare multi-system genetic disorder.
Case Report
A 12-year-old girl was referred to our tertiary children’s hospital for the diagnostic work up of ILD and possible lung transplantation. Her previous medical history is significant for respiratory distress since her third year of life with recurrent episodes of pneumonia. At about 3-4 years of age, she presented with a right pneumothorax which required treatment with blebectomy and pleurodesis. At 12 months of age, a hemodynamically significant patent ductus arteriosus (PDA) was closed. One year before the admission to our children’s hospital, a biopsy of the left lung was performed because of her worsening respiratory distress. The results were suspicious of pulmonary lymphangioleiomyomatosis (LAM). A Chest CT (Figure 1) showed a global distortion of the pulmonary architecture, mild central bronchiectasis, moderate bronchial wall thickening, septal thickening and predominant hyperinflation of the upper lobes while the lower lobes appeared atelectatic. A follow up chest CT showed stable findings. The CT study also showed thoracic vertebral body changes (Figure 2), including decreased antero-posterior dimension of the upper thoracic vertebral bodies and mild anterior wedging of the mid and lower thoracic vertebral bodies. MRI of the brain (Figures 3 & 4) revealed a moderate right dominant widening of the lateral ventricles, a normal sized third and fourth ventricle, chronically ruptured leaves of the septum pellucidum, thinning and stretching of the corpus callosum and extensive contiguous bilateral periventricular nodular gray matter heterotopia (PNH).
Figure 1:Coronal (A) and serial axial chest CT images with lung windows of the upper (B), middle (C) and lower (D) lobes demonstrating interstitial lung disease changes. Upper lobe and mid lung predominant hyperinflation are noted bilaterally. There are ground glass opacities in bilateral central para-hilar zones predominantly on the lower lobes (arrows). In addition, mild to moderate septal thickening is predominantly seen in the bilateral lower lobes. Bilateral central mild bronchiectasis without mucus plugging and moderate bronchial wall thickening are also noted.
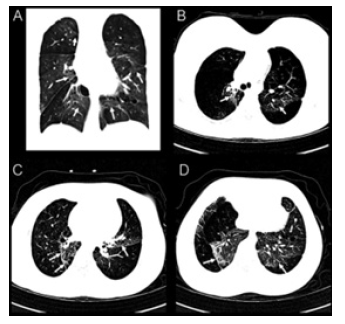
Figure 2:Sagittal CT scan of thoracolumbar spine showing thoracic vertebral body changes, including decreased antero-posterior dimension in the upper thoracic bodies and mild anterior wedging of the mid and lower thoracic vertebral bodies.
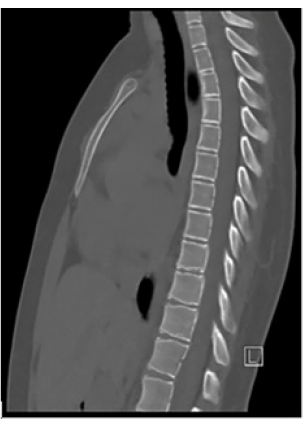
Figure 3:Axial T1WI (A), T2WI (B) and FLAIR (C) MRI of the brain, showing moderate lateral ventriculomegaly greater on the right side and numerous foci of periventricular nodular gray matter heterotopias along the right and left ventricles (arrows).
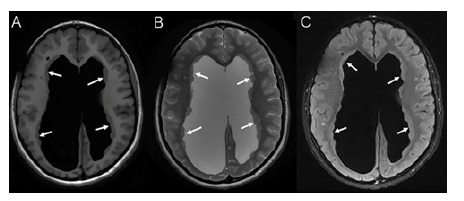
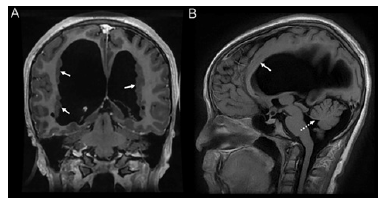
Figure 4:Coronal T1WI (A) MRI of the brain showing moderate lateral ventriculomegaly greater on the right side and numerous foci of periventricular nodular gray matter heterotopias along the right and left ventricles (arrows). Sagittal T1WI (B) MRI of the brain demonstrating mild hypoplasia of the inferior cerebellar vermis (round dot arrow) and thinning of the corpus callosum (arrow).
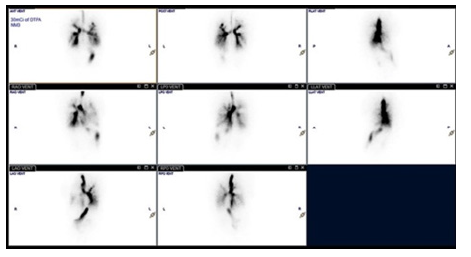
Based upon the combination of the lung abnormalities (ILD, recurrent pneumonia), cardiac anomaly (PDA), and the brain MRI findings (PNH), the suspicion for FLNA related pathology was raised. The subsequent genetic work up confirmed final diagnosis. Additional diagnostic examinations included spine radiographs demonstrating a 20-degree scoliosis curvature (Figure 5) and DEXA scan with low bone density for age and gender. On admission to our hospital, the physical examination revealed impaired gross motor skills, core weakness and postural instability. Her daily activities were limited due to significant dyspnea on exertion with desaturations to mid-70s while ambulating. Despite the fact that the patient was cleared for lung transplantation, she and her parents decided against the procedure. In the following months, the child’s condition deteriorated with worsening respiratory distress. A nuclear medicine lung ventilation/perfusion scan (Figure 6) identified large, irregular ventilation defects affecting predominantly the right greater than left bilateral upper lobes which correlated well with the pulmonary parenchymal disease distribution as seen on the previous CT.
Figure 5:Spine XR EOS frontal view (A) showing right convex scoliosis with apex at T10 of 20 degrees and left convex scoliosis with apex at L2 of 16 of degrees; lateral view (B) showing lumbar lordosis that measures 70 degrees.
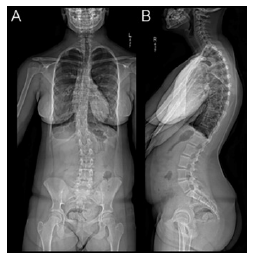
Figure 6:Serial ventilation lung scans showing large, irregular ventilation defects affecting predominantly the right greater than left bilateral upper lobes. Perfusion scan (not shown) was normal.
Discussion
The FLNA gene encodes for the protein filamin A, an actin linking protein which has an important role in building the internal network of protein filaments in the cell cytoskeleton. Therefore, mutations of this gene impact the development of multiple organs leading to PNH, ILD, skeletal dysplasia, and vascular and cardiac abnormalities [5]. FLNA related pathology occur mainly in females because PNH is an X-linked dominant inherited disorder and when present in hemizygosity, provoke early abrogation of male intrauterine development leading to early death [2,8]. The first case of interstitial lung disease associated with FLNA mutation was published in 2009 [2] and since then, a total of 30 cases have been described in the literature.
Pulmonary symptoms occur during childhood, and the lack of pulmonary involvement in adults is due to early death or lung transplantation [8]. In terms of treatment, the most severe phenotype with progressive respiratory failure typically requires lung transplantation; in some cases, lung volume reduction surgery may represent an alternative option [3]. The first familial case of emphysema in two adults with a LOF mutation of the FLNA gene was presented in 2021 by Valentin et al. [8]. Correct diagnosis is often delayed due to the lack of general knowledge of this disease and the presence of milder phenotypes [7].
Similarly, correct diagnosis was challenging in our patient, likely due to a combination of an initial mild clinical presentation and rarity of the disease. The performed chest CT was in concordance with ILD findings described in literature and showed global architecture distortion, areas of pulmonary atelectasis, hyperinflation, septal thickening, mild central bronchiectasis, bronchial wall thickening. Among the broad range of cardiac abnormalities associated with FLNA mutation, atrial septal defects, PDA, valve abnormalities, aortic root dilatation and aneurysms have been described [5]. Our patient apart from a PDA successfully occluded, did not present with other cardiac issues. Skeletal alterations are usually associated with GOF mutations and in most cases include diseases of the OPDSD. Exceptionally, as in our case, milder osseous alterations may be described in LOF mutations [2]. In our patient, the skeleton was only mildly affected, characterized by mild scoliosis, thoracic kyphosis, and rounded shoulders. The most striking alterations were noted on the brain MRI. She presented with extensive PNH, a classic example of a developmental disorder secondary to a filamin A deficiency. Reported neuroimaging findings include multiple, bilateral, usually symmetric, subependymal foci of nodular gray matter heterotopia, an enlarged cisterna magna and mild cerebellar vermis hypoplasia. PNH usually presents with epilepsy and is often associated with mild intellectual disability and learning difficulties. Despite the extensive PNH and mild ventriculomegaly, our patient did not suffer from seizures. Neurological sequelae did however include impaired gross motor skills, mild core weakness and postural instability or other neurodevelopmental consequences.
Conclusion
The limited literature related to the FNLA mutations and the phenotypic variability of clinical presentation poses challenges for an early and correct diagnosis. We report on this patient to alert radiologists and neuroradiologists that when faced with the combination of ILD and PNH in a child, FLNA mutations should be considered in the differential diagnosis and a targeted genetic work up should be performed for a definitive diagnosis confirmation.
Statement and Declarations
Conflict of interest: The authors have no relevant financial or non-financial interests to disclose.
Funding information: No funding was received to assist with the preparation of this manuscript.
Ethics approval: Approval was not necessary for this kind of manuscript.
Consent to participate: Consent was not necessary for this kind of manuscript.
References
- Sasaki E, Byrne AT, Phelan E, Cox DW, Reardon W (2019) A review of filamin A mutations and associated interstitial lung disease. Eur J Pediatr 178(2): 121-129.
- De Wit MC, Kros JM, Halley DJ, De Coo IFM, Verdijk R, et al. (2009) Filamin a mutation, a common cause for periventricular heterotopia, aneurysms and cardiac defects. J Neurol Neurosurg Psychiatry 80(4): 426-428.
- Burrage LC, Heinle JS, Cerfolio RH Robert PG, Kalyani RP, et al. (2022) Application of lung volume reduction surgery for a child with filamin A (FLNA) mutations. Pediatr Pulmonol 57(1): 224-230.
- Lord A, Shapiro AJ, Saint MC, Claveau M, Melancon S, et al. (2014) Filamin a mutation may be associated with diffuse lung disease mimicking bronchopulmonary dysplasia in premature newborns. Respir Care 59(11): e171-e177.
- Ieda D, Hori I, Nakamura Y, Hironori O, Yutaka N, et al. (2018) A novel truncating mutation in FLNA causes periventricular nodular heterotopia, Ehlers-Danlos-like collagenopathy and macrothrombocytopenia. Brain Dev 40(6): 489-492.
- Zhou AX, Hartwig JH, Akyurek LM (2010) Filamins in cell signaling, transcription and organ development. Trends Cell Biol 20(2): 113-123.
- West T, Williamson N, Akhter J (2023) Case report: Filamin A mutation lung disease recognized in an 11-year-old child. Pediatr Pulmonol 58(1): 61-65.
- Valentin V, Bervar JF, Vincent DC, Thomas S, Lidwine W, et al. (2021) Filamin a mutations: A new cause of unexplained emphysema in adults? Chest 159(3): e131-e135.
© 2023 Livja Mertiri. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






