- Submissions

Full Text
Research in Pediatrics & Neonatology
Replacement of the Ureter with a Pelvic Flap in an Infant with Congenital Total Ureteral Stenosis
Shchapov Nikolay1,5*, Ekimovskaya Ekaterina2,5, Shatova Svetlana3,5, Sergeyeva Svetlana4,5 and Kulikov Denis1,5
1Thoracoabdominal surgery service and emergency surgical care for children, Ilyinskaya Hospital, Russia
2Surgical Department of Newborns and Infants, The National Medical Research Center of Children’s Health, Russia
3Pediatric department, MEDSI clinic in Kotelniki, 5, Sosnovayast, Kotelniki, Lybertsydistr, Russian
4V.F.Voyno-Yasenetsky Scientific and Practical Center of Specialized Medical Care for Children, Russia
5Department of Neonatal Surgery, Moscow Regional Center for Maternity and Childhood Healthcare, Russia
*Corresponding author: Nikolay Shchapov, Thoracoabdominal surgery service and emergency surgical care for children of the Ilyinskaya Hospital, build. 2, 2, Rublevskoepredmestiest., vil. Glukhovo, Moscow Region, Russia
Submission: February 07, 2023; Published: March 09, 2023

ISSN: 2577-9200 Volume7 Issue3
Abstract
The combination of unilateral renal ectopia with congenital total ureter stenosis is a very rare
malformation. If renal function is safe, the reconstruction of the upper urinary tract can be challenging.
We would like to present our experience of unilateral ureter reconstruction using part of renal pelvis and
sliding triangular flaps technique.
Materials: From 2018 to 2021, 454 children with congenital urinary tract malformations were treated
in our department. Supraspinal obstruction was diagnosed in 133 patients and required surgery:
laparoscopic platy of ureteropelvic junction (n=47), endoscopic stents of narrowed distal part of ureter
(n=74), platy of extravesical part of ureter because of stenosis or membrane (n=2). We performed reimplantation
of ureter in 16 cases: stenting was of no effect (n=4), stent was impossible to place due
to stenosis (n=4), bladder neck (n=7) and urethra (n=1) ureter ectopia. Total ureter stenosis was
intraoperatively diagnosed in one child with renal ectopia and supraspinal obstruction, so it was
impossible to reimplant the ureter. Since the kidney had normal function and was located behind the
bladder, we performed ureter reconstruction using part of renal pelvis and sliding triangular flaps
technique. We had enough tissue to lengthen the pelvis to the bladder easily. Also, this maneuver allowed
us to create an anti-reflux mechanism.
Result: There were no complications. Ultrasound follow-up showed no obstruction of the upper urinary
tract.
Conclusion: In case of renal ectopia and total ureter stenosis, urethroplasty with a pelvic flap using
sliding triangular flaps technique may be the operation of choice if the kidney function is normal.
Keywords:LCongenital ureter stenosis; Renal ectopia; Re-implantation of the ureter; Pelvis lengthening; Sliding triangular flaps; Children
Abbreviations:US: Ultrasound Examination; 99mTc: Technetium-99m; CT: Computed Tomography; RP: Radiopharmaceutical; DMSA: Dimercaptosuccinic Acid
Background
Renal pelvic ectopia is not common and does not require any treatment in most cases. The anomaly can be explained as a variant of incomplete embryogenesis. When we suspect an alteration of kidney development mechanisms we may expect kidney malformations in combination with renal ectopy to appear more often, but in practice it is not true. From 2018 to 2021, 454 children with various congenital malformations of urinary tract were treated in our department. Laparoscopic correction of hydronephrosis was performed in 47 children, endoscopic stenting of narrowed distal part of ureter was performed in 74 children. Obstruction of the middle third of the ureter was detected in 2 children, which required platy of this segment. Ureter re-implantation was required in 16 children: in 4 cases our stenting was of no effect, in 4 cases stent was impossible to place due to stenosis and in other cases we found ureteral ectopia in the bladder neck (7 children) or urethra (1 child). One patient had renal ectopia together with supraspinal obstruction because of total ureteral stenosis. We would like to present this case.
Method and Materials
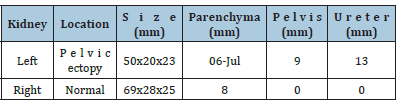
A full-term girl (aged 3 days) with antenatally diagnosed left kidney pelvic ectopia and dilation of collecting system was admitted to our department with urinary tract infection. Examination included ultrasound (US), retrograde fictional cystourethrography and revealed a left reflux-free megaureter. Cystourethroscopy showed dystopia and stenosis of the left ureter. We placed a stent into the left ureter to decompress the kidney and control urine production with further intention to check its function. Conservative anti-inflammatory therapy helped to cure infection, and the child was discharged home. Six weeks later, the stent stopped urinating and the child had a fever again. We flushed out the stent and added one more course of anti-inflammatory therapy. Unfortunately, in the two next weeks the stent was accidentally removed. Then the patient was admitted to our department at the age of 4 months. Preoperative examination included renal US, static nephroscintigraphy (SNS) and excretory urography. US showed pelvic ectopia of the left kidney, smaller size compared to the right one, loss of corticomedullary differentiation, but normal parenchyma (Table 1). We found normal blood supply to the left kidney (both Doppler and energy mode). US findings suggested that we saw left kidney pelvis (up to 9mm) and left ureter (up to 13mm). SNS with Technetium-99m (99mTc) showed dystopia of the left kidney, diffuse-focal changes of parenchyma, and loss of function by 60%. The right kidney had normal function with moderate vicarious hypertrophy. Parenchyma overall function was reduced by 10%. Excretory urography, unfortunately, gave no additional information to specify the anatomy of the malformation. Retrograde pyelography could be an option to provide for details, but the stent was accidentally removed before. The simplest way suggested unilateral nephroureterectomy because of complex anatomy, inadequate visualization in urography, and US findings of left kidney hypoplasia. However, almost normal thickness of parenchyma and 40% of kidney function were indications for organ-preserving surgery. As we saw the left ureter sized of 13mm we chose trans vesical resection of the narrowed ureter and Cohen re-implantation.
Table 1: Preoperative renal ultrasound.
We performed an open surgery with Pfannenstiel access. The revision of the bladder showed the left ureter orifice located very close to the bladder neck. Our attempt to stent the ureter failed as at the distance of 2cm we met stenosis. We mobilized the intravesical part of the ureter using electrocoagulation and dissected it from the retro vesical space. The ureter was like a scar cord, it was difficult to isolate it. We dissected the bladder mucosa and detrusor 1.5cm upwards from the ureteral entry to ensure its safe mobilization. The length of the mobilized ureter was about 5cm, its diameter was 2mm with many areas of stenosis up to the ureteropelvic junction. The left kidney was located behind the bladder, its pelvis was 4x4cm in size. Thus, it was impossible to perform re-implantation. Taking into account the level of the left kidney function, pelvic dystopia and significant pelvic dilatation, we decided to perform urethroplasty with a pelvic flap using sliding triangular flaps technique. We elongated the pelvis with sliding triangular flaps and narrowing of the distal segment (Figure 1). Doing so we made a tubular structure 7-8cm long and about 5mm in diameter. Then we formed a 2cm tunnel in the submucosal layer of the bladder and put the distal part of neo ureter through it. We fixed the pelvis to the detrusor right at the bladder way out, sutured detrusor and mucosa layer, so the posterior wall of the bladder was restored. Finally, we placed a stent into neo ureter and pelvis (Figure 2), pulled it out through a separate puncture on the abdomen wall, put a Foley catheter Ch. 10 and sutured the anterior wall of the bladder.
Figure 1: Scheme of elongation of the pelvis with sliding triangular flaps: I-Scheme of dissection of the pelvis (1-anterior wall, 2-posterior wall, marking angles A-O, l -length to plastic), II-Scheme of moving triangles (marking angles A-O), III-Final suture of the pelvis (marking the corners A-O, 2l - length after plastic surgery).
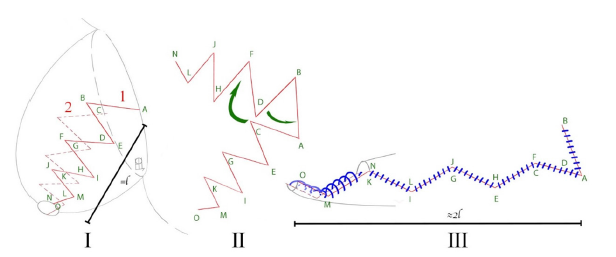
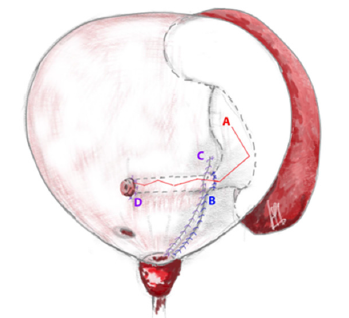
Figure 2: Scheme of the anatomy after operation (A-suture of the pelvis, B-suture of the detrusor with fixation of the pelvis, C-suture of the mucosa, D-mouth of the neourethra).
Results
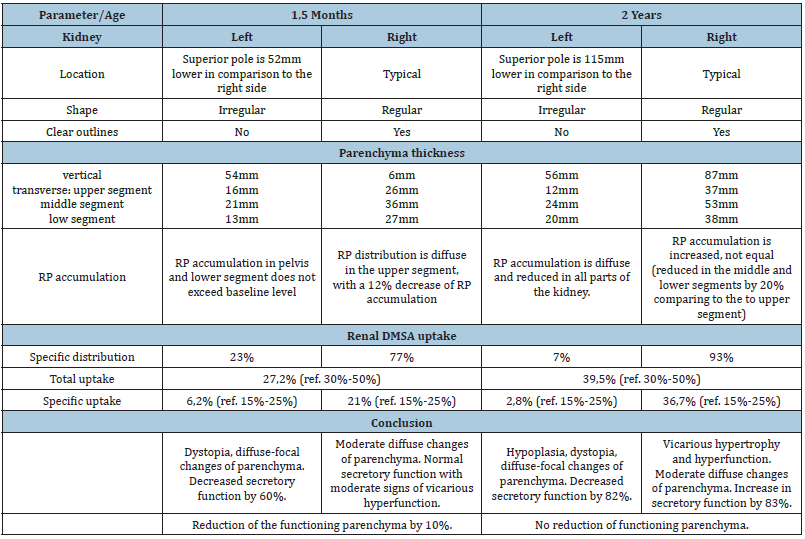
The operation time was 3 hours, blood loss was minimal, there were no intraoperative complications. Urine began to drain through the stent immediately after the operation. On the first postoperative day the urine volume from the left kidney was twice more as from the right one. Since the second postoperative day both kidneys gave equal amounts (1.5ml/kg/day). Hematuria was observed up to the 6th day after surgery, but routine hemostatic therapy helped and the child had no anemia. The Foley catheter was removed on the 7th postoperative day, the child urinated spontaneously, no dysuria was observed. The control US showed smaller size of the left kidney (41x18x19mm versus 50x20x23) with no changes in parenchyma thickness (6-7mm). We performed pyelography on the 13th day after the operation (Figure 3): as the pelvis was filled, dilated calyces appeared, the pelvis narrowed conically while entering the bladder, the contrast flowed into the neourethra around the stent. Neither neo ureter dilatation nor leakage were found. After we removed the stent we found a small urinary leakage which resolved spontaneously. The final US showed the left kidney reduced to 36x18x19mm, the pelvis was 4mm, and the same parenchyma thickness. The child was discharged home on the 20th postoperative day. US follow-up showed no pelvic dilatation or loss of parenchyma thickness. After one year the child moved to another city and obtained medical care in some other medical clinic. All the information we could get from her parents was that SNS showed no renal function at the age of 2 years (Table 2), and the girl underwent unilateral nephroureterectomy of the left kidney.
Figure 3: Postoperative contrast pyelography (A-pelvis, B-calyces, C-stent).
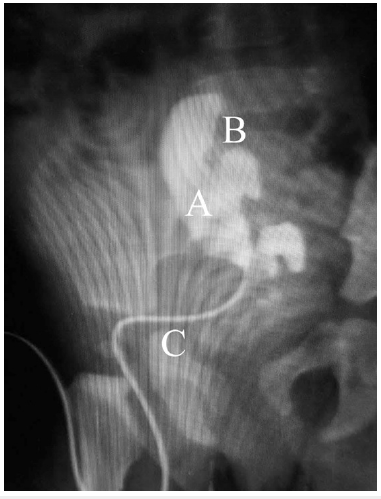
Table 2: Comparison of the results of static renal scintigraphy before surgery and at the age of 2 years. RPradiopharmaceutical (99mTc), DMSA – dimercaptosuccinic acid radiolabeled with technetium-99m.
Discussion
There are few publications which describe patients with ectopic kidneys in combination with malformations of the upper urinary tract in recent literature. The most detailed study was published by Philip E Gleason et al. [1] with 77 patients aged from 1 day to 39 years for the period of 70 years. In our cohort of patients with various urinary tract anomalies, ectopic kidney occurred only in one child, so we report this case. Congenital total stenosis of the ureter is a very rare variant of malformation. We find only one case similar to ours [2]. Ureter disorders that require replacement are more common in cancer patients, or with severe vesicoureteral reflux, when the ureter loses its contractility and is unable to divert urine from the kidney due to its extreme dilation. Usually, the ureter is replaced by ileum tissue [3,4] or appendix [2], or by a vein (an experimental study by Wolters [5] and Dal Moro [6]). We found one paper describing the use of a pelvic flap in an adult patient with ureteropelvic junction obstruction and kidney ectopia, when the ureter was short and deformed by adhesions. The authors reduced the pelvis and created a tubular pelvic flap laparoscopically using the Endo-GIA neourethra device [7]. In our case, we considered using ileum tissue to provide urine outflow from the pelvis, but the high risk of infectious complications, the need to involve the abdominal cavity influenced the final choice of surgical tactics. We summed up our experience, the paper published by Giuseppe Simone et al. [7] and our discussion with other urologists of Ilyinskaya Hospital, who performed a robotic ureter platy using pelvic flaps in adult patients. So, we came to the conclusion that replacement of ureter (total or partial) with a pelvic flap is a good practice to avoid the use of intestine. The operation can be performed laparoscopically or robot-assisted.
Retrospectively, we suggest that children with various upper urinary tract malformations should undergo CT with volumetric reconstruction of kidneys and urinary tract, magnetic resonance imaging and antegrade or retrograde pyelography (if possible) in addition to renal US. Diuretic prosopography, radionuclide renography, and functional magnetic resonance urography are necessary to get information about renal function [8]. Each diagnostic method has its advantages and limitations, and the surgeon’s task is to use all the advantages to obtain the most complete information about the anatomy, syntropy, and functional state of the affected organ. Comparing CT to secretory urography (or intravenous pyelogram), we should notice that CT provides not a planar, but a three-dimensional image which gives detailed anatomy of the collecting system, vascular and soft tissue features. Secretory urography is mainly used to evaluate anatomy of upper urinary tract and kidney function in dynamics. We also considered the idea to perform CT after excretory urography [9]. Obviously, it can provide details about upper urinary tract, but not vessels. So, in our practice, we use CT with intravenous contrast enhancement and obtain a native scan, arterial, parenchymal and venous phases, as well as early excretory (5 minutes after contrast administration) and late excretory (90 minutes) phases. The most informative method to understand kidney function is renal scintigraphy, which is based on accumulation of a radiopharmaceutical (99mTc) in the kidney parenchyma. This technique is aimed to see excretion and distribution of the radiopharmaceutical in each kidney [10]. Also, this method can be used to evaluate if the kidney function has recovered after treatment.
To avoid urine leakage, we put suture to bladder wall around the stent. But after 1 week the stent starts to form a fistula, so if we remove it after one week-we may have urine leakage. In such cases we do not close the fistula but wait until it resolves spontaneously. Otherwise, the fistula can lead to an abscess. Of course, as a result, we see an unfavorable outcome-loss of kidney function, which required unilateral nephroureterectomy. Unfortunately, we don’t have morphology of resected kidney to explain why it happened, but according to our experience we can suppose several mechanisms. Firstly, it could be congenital malformation of parenchyma, such as renal cystic dysplasia. It is a rather common anomaly which can be genetically determined also [11]. Since renal glomerulus and collecting system develop from different germs, they act separately in such type of malformation. The glomerulus produces primary urine, but if there is no connection with renal tubule, urine accumulates and forms a cyst. It grows and compresses vessels so glomerulus atrophies. Subsequently, primary urine resolves and glomerulus turns into scar tissue. If the kidney produces urine for some time, this means that some nephrons developed normally. However, cysts compress not only capillaries, but also affect surrounding tissues. It is not possible to measure to what extent the kidney was damaged, as well as predict possible atrophy of damaged glomeruli. The only thing we can definitely admit is that compression of kidney parenchyma by blocked urine outflow or urinary tract inflammation increase the actual damage of compromised glomeruli. As a result, we see constant loss of kidney function as the child grows up.
Secondly, we can observe such damage of kidney parenchyma when it is critically compressed (severe hydronephrosis or megaureter) or constantly injured (vesicoureteral reflux). If the damaging agent is delayed to cure, focal segmental glomerular sclerosis may develop resulting in scarring of the parenchyma. Thirdly, blood supply may be lacking. As the kidney has hypoplasia and ectopia we can suspect it had abnormal vessels and capillaries. Child growth, compression by bladder (when it is full) or maybe both factors could worsen kidney blood supply, which eventually led to function loss. One can question if the kidney function was already lost by the time of the operation? Did the ureter reconstruction ensure effective urine outflow? The answer is-urine production. We saw urine outflow through the stent immediately after the operation and further on, which meant that the kidney did function. Our experience of nephrostomy in severe hydronephrosis with loss of function proved the same. During the first few days after the decompression urine run off, but very fast the outflow faded away and starting from the 5th postoperative day it was 30-50ml per day. The patient we report about had a stent for almost two weeks with a diuresis rate similar to her healthy kidney. When we removed the stent urine outflow did not lead to pelvis dilation, which proved no neourethra dysfunction (US showed even decreased size of the pelvis). Therefore, nor preoperative loss of function neither postoperative neo ureter obstruction can explain bad outcome.
So, what are the markers and predictors of irreversible kidney damage? How to choose the best treatment in such cases? Of course, many surgeons stand for the age limit of 3-6 months for a child to be operated on. Some of them wait up to 1 year if only one kidney is affected. Our approach suggested that early correction allowed us to save and restore kidney function. But still, detailed criteria need to be specified. In our case reconstructive surgery was justified paying attention to normal urine production and 40% of function according to renal scintigraphy. Our experience of reconstructive operations in infants proved that they had a great rehabilitation potential even with severe hydronephrosis, but with normal kidney function. Therefore, operational risks can hardly justify nephroureterectomy if kidney function is more than 15%. For example, in the article published by Haitham Dagash et al. [2], the authors performed reconstructive operation in a child with total congenital ureteral stenosis and 30% of kidney function and the outcome was good.
What would the criteria be for nephrectomy in addition to kidney function? Of course, we think of kidney hypoplasia, but ectopic kidney is often smaller than the one with typical location. Noteworthy is the absence of RP accumulation in the left kidney pelvis. In a child with supraspinal obstruction, blocked urine outflow, but its normal production the pelvis would accumulate isotopes. But in our case it didn’t happen. The possible reasons were: urine did not contain isotopes as it was blocked in the pelvis; kidney parenchyma was of embryonic renal tissue. Similar picture we see in multicyclic dysplastic kidneys when rudimentary blood flow supports primary urine production and no spontaneous regression of the damaged kidney occurs. However, the important criteria for kidney function loss (even if the embryonic tissue accumulates RP) are vicarious hyperfunction and hyperplasia of the healthy kidney. We did not see any at the time of our operation. But these signs were present when the affected kidney lost its function. Nephrostomy can be an alternative, but pelvic ectopia made the risks of vessels and abdomen organs injury high enough. Also, one should consider how long the child will carry nephrostomy tube until the necessary surgery is performed. Thus, our decision was to choose urethroplasty with a pelvic flap using sliding triangular flaps technique.
Conclusion
The presented case turned out to be unique in our practice. Our experience showed that urethroplasty with a pelvic flap may become the operation of choice for total or partial ureteral replacement. Despite the pelvic structure and peristalsis differ from the ureter, it will provide for adequate evacuation of urine.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors have no conflicts of interest to declare.
References
- Gleason PE, Kelalis PP, Husmann DA, Kramer SA (1994) Hydronephrosis in renal ectopia: Incidence, Etiology and Significance. The Journal of Urology 151(6): 1660-1661.
- Dagash H, Sen S, Chacko J, Karl S, Ghosh D, et al. (2008) The appendix as ureteral substitute: A report of 10 cases. Journal of Pediatric Urology 4(1): 14-19.
- Zhong W, Du Y, Yang K, Meng S, Lin R, et al. (2017) Ileal ureter replacement combined with boari flap-psoas hitch to treat full-length ureteral defects: Technique and initial experience. Urology 108: 201-206.
- Bayne CE, Cardona GD, Hsieh MH (2017) Robotic versus open pediatric ureteral reimplantation. Journal of Pediatric Urology 13(2): 128-129.
- Wolters HH, Heistermann HP, Stöppeler S, Hierlemann H, Spiegel HU, et al. (2010) A new technique for ureteral defect lesion reconstruction using an autologous vein graft and a biodegradable endoluminal stent. The Journal of urology 184(3): 1197-1203.
- Dal MF, Macchi V, Porzionato A, Mandato FG, De Caro R (2019) RUG technique: Replacement of the ureter with gonadal vein. A cadaveric study. Minerva Urol Nefrol 71(1): 85-91.
- Simone G, Leonardo C, Papalia R, Guaglianone S, Sacco R, et al. (2007) Case report: Laparoscopic ureteral reconstruction with pelvic flap in ureteropelvic junction obstruction of ectopic left kidney. Journal of endourology 21(9): 1041-1043.
- Nguyen HT, Benson CB, Bromley B, Campbell JB, Chow J, et al. (2014) Multidisciplinary consensus on the classification of prenatal and postnatal urinary tract dilation (UTD classification system). Journal of pediatric urology 10(6): 982-998.
- Hu H, Hu XY, Fang XM, Chen HW, Yao XJ (2009) Unenhanced helical CT following excretory urography in the diagnosis of upper urinary tract disease: A little more cost, a lot more value. Urological Research 38(2): 127-133.
- Biassoni L, Easty M (2017) Paediatric nuclear medicine imaging. British Medical Bulletin 123(1): 127-148.
- Devlin LA, Sayer JA (2019) Renal ciliopathies. Current Opinion in Genetics & Development 56: 49-60.
© 2023 Nikolay Shchapov. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






